Research - Electronic Structure |
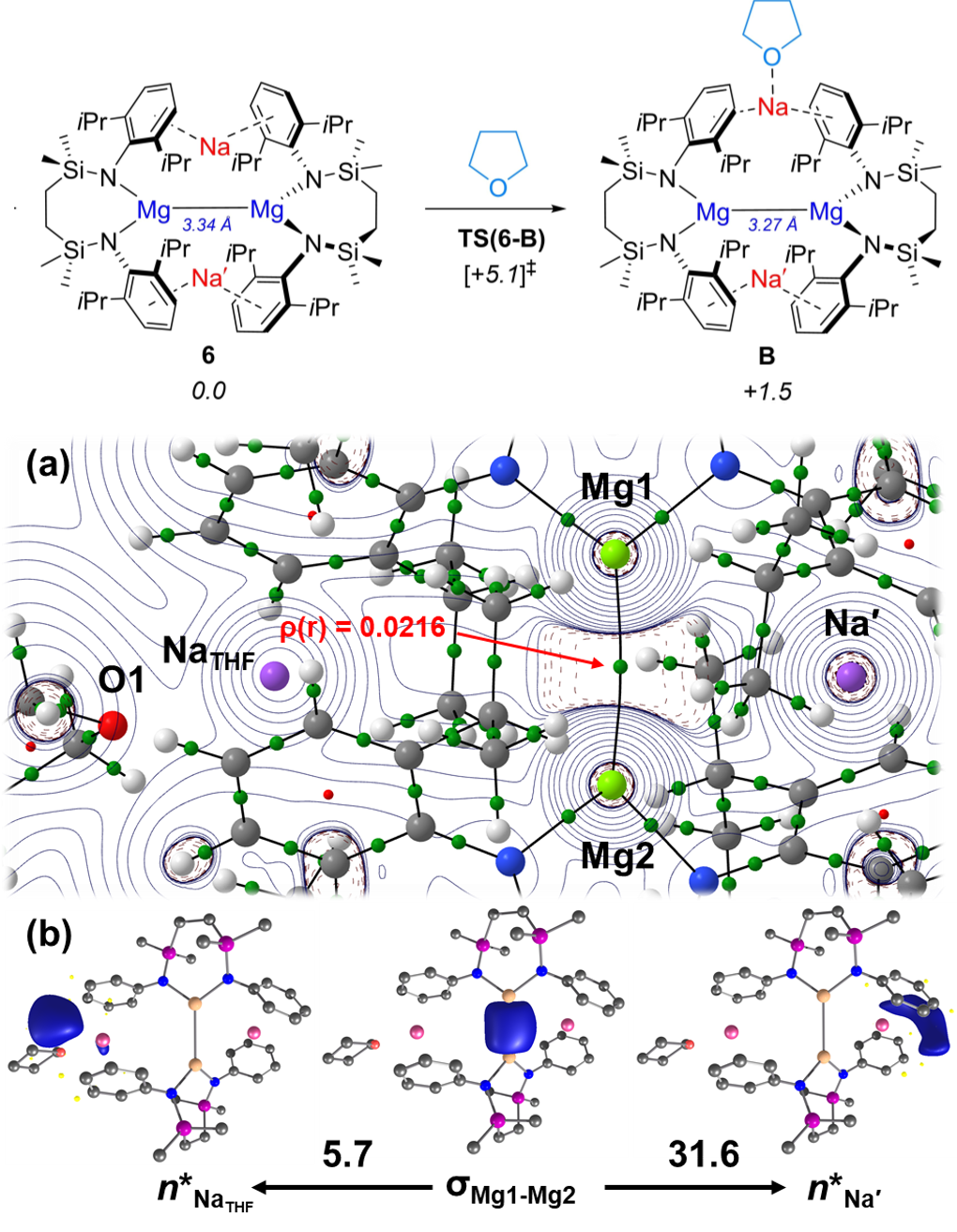 |  |
Alongside mechanistic modelling of reaction systems, we employ a range of electronic structure techniques to add further value and understanding to the structures, intermediates and transition states we have computed. These techniques include Natural Bond Orbital (NBO) analysis, Quantum Theory of Atoms in Molecules (QTAIM) and Energy Decomposition Analysis (EDA) approaches. As the images above show (description below), a combination of approaches can work in partnership to illuminate interesting features and provide great context for why certain reactivity is observed. Based on experimental data from the Hill group, we mechanistically explored the coordination of a THF solvent molecule to a sodium cation of the dimagnesium complex 6. We were able to identify a barrier of 5.1 kcal mol-1 for the association of THF to the sodium, to give intermediate 8 with a free energy of 1.5 kcal mol-1. QTAIM data highlighted increased electron density between the non-coordinated sodium cation and the Mg-Mg bond, including pronounced curvature of the Mg-Mg bond path to this sodium atom. Additionally, bond orbitals from NBO data could visualise the interactions between the Mg-Mg sigma bond and the sodium cations, with second order perturbation energies confirming the reduction in interaction for the THF-coordinated sodium to only 5.7 kcal mol-1, whilst the non-coordinated sodium saw an increase in interaction with the Mg-Mg sigma bond to 31.6 kcal mol-1. See the 2023 publication in Angewandte Chemie for further details. |