Fuel Cells
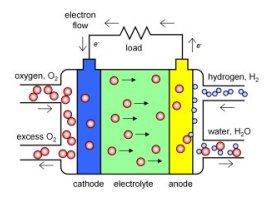
The concept of a fuel cell was first demonstrated by William Grove in 1839 (with some early results published in the Proceedings of the Royal Society). But what is a fuel cell? Like a combustion engine, a fuel cell uses some sort of chemical fuel (such as hydrogen). But like a battery, the chemical energy is converted directly to electricity, without the polluting combustion step.
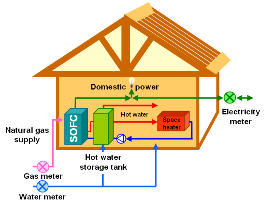 Polymer-based fuel cells are being developed for cars (and London buses!) while solid oxide fuel cells are suitable for heat and power generation in
homes. During the 2003 New York blackout the lights only stayed on in the few buildings powered by fuel cells. But there are still major challenges for
fuel cell technology. In particular, new ion conducting materials hold the key to better and cheaper solid oxide fuel cells at lower temperatures.
Polymer-based fuel cells are being developed for cars (and London buses!) while solid oxide fuel cells are suitable for heat and power generation in
homes. During the 2003 New York blackout the lights only stayed on in the few buildings powered by fuel cells. But there are still major challenges for
fuel cell technology. In particular, new ion conducting materials hold the key to better and cheaper solid oxide fuel cells at lower temperatures.
 At Bath, we use supercomputers to build atomic-scale models helping us understand and design new crystalline
and nanostructured oxides that can
be tested in the laboratory. Promising alternatives to current fuel cell materials include novel apatite compounds that show fast-ion
conduction through their beautiful crystalline structures. Computer modelling has allowed us to gain fresh insight into ion motion in solids which is
difficult to extract from experiment alone. This could lead to the design of better solid oxide fuel cells in the future.
At Bath, we use supercomputers to build atomic-scale models helping us understand and design new crystalline
and nanostructured oxides that can
be tested in the laboratory. Promising alternatives to current fuel cell materials include novel apatite compounds that show fast-ion
conduction through their beautiful crystalline structures. Computer modelling has allowed us to gain fresh insight into ion motion in solids which is
difficult to extract from experiment alone. This could lead to the design of better solid oxide fuel cells in the future.
Looking ahead, a major goal for polymer fuel cell development is their widespread use in the transport industry. A fuel cell car could run on hydrogen, which can be derived from renewables such as solar power, and the only by-product would be water.
|
Watch an animation of how a fuel cell works! Press the image to begin (2.4 Mb) |

|
The Fuel Cell Team
Prof. Saiful Islam, Dr Craig Fisher, Dr Alison Jones, Julia Percival.
More Information
- Download the fuel cell fact sheet (1.8 Mb).
- Visit the Islam Research Group web site.


