|
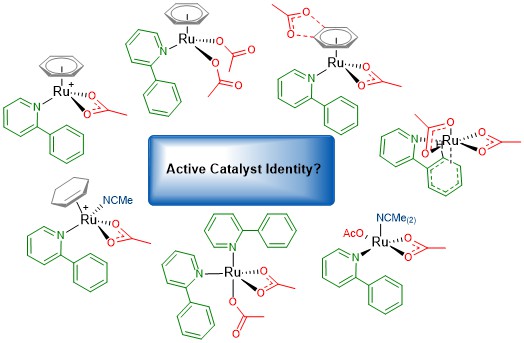
C-H Activation The impact of breaking a C-H bond is at the forefront of the research area of C-H activation. Our specific interest lies in the form the catalyst structure takes during the reaction process and what the active mechanism is. When using a carboxylate base to activate a directing group (DG) C-H bond there are several theorised mechanisms that could be operating. Our most recent paper (DOI: 10.1039/c9ob01092k) explores the consequences for a Ru catalyst system when acetonitrile is the experimental solvent. The ability of the acetonitrile (MeCN) molecules to coordinate as a ligand to the ruthenium centre, and influence the coordination sphere at Ru was examined and the potential shown for dissociation of the arene co-ligand in these experimental conditions. Recent Publications:
|