Why develop a chemical sensor for nitrous oxide?
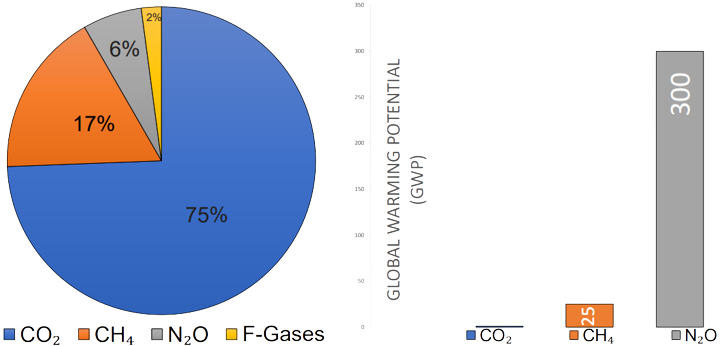
Nitrous oxide, colloquially known as laughing gas, has the chemical formula N2O. It is often confused with the NOx gases however is notably different! There are many contributing man-made greenhouses gases to climate change, however, only a few crucial gases make up most of the climate change effect. The top three are carbon dioxide, methane, and nitrous oxide. Currently nitrous oxide contributes six percent to the man-made green house gas effect. Alarmingly though it is three hundred times more contributing per molecule than CO2!
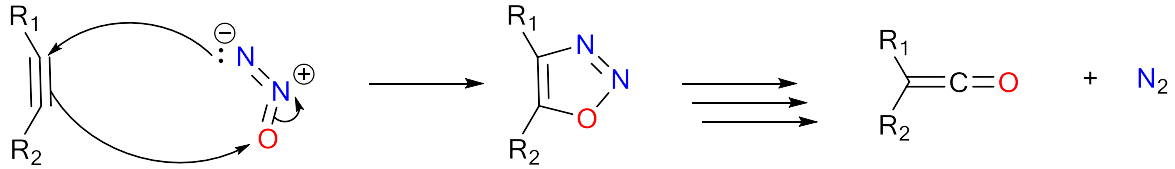
Developing a chemical sensor for nitrous oxide
Nitrous oxide poses a problem for chemical sensing, the molecule is very inert and undergoes very few chemical reactions. Fortunately, it can undergo a 3+2 cycloaddition with an alkyne, this currently requires forcing conditions. After the 3+2 cycloaddition there are multiple intramolecular reactions, these eventually result in the loss of nitrogen gas, and creation of a reactive ketene species. The ketene will react intramolecularly depending on the R1 and R2 groups.