
New Rate Expressions for Assisted Catalyst Design
Dr. Olivier Camus and Prof. Stan Kolaczkowski
Department of Chemical Engineering, University of Bath, Claverton Down, Bath, BA2 7AY, UK
And in collaboration with Pr. Robbie Burch and Dr. James A. Sullivan
The School of Chemistry,David Keir Building, Stranmillis Rd., The Queens University of Belfast, Belfast, BT9 5AG, County Antrim, Northern Ireland
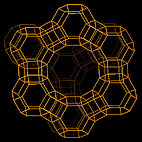
Today, most of the reaction rate calculations are based on power law or Langmuir-Hinshelwood expressions. This study aims to bridge a gap between chemist and chemical engineers in developing new rate expressions incorporating the catalyst properties. These new rate expressions consist of groups of parameters that quite distinctly describe the rates of adsorption and desorption of individual species and the rates of intrinsic reactions. To determine these parameters, the catalyst properties, like pore size distribution, number of acid sites, have been measured. The reaction selected for this project is the vapour phase synthesis of methyl tert-butyl ether from isobutene and methanol. Experiments were performed in a steady state micro reactor at atmospheric pressure. For stoichiometric mole ratios, reactions at temperatures between 60 and 120°C were studied. Other experiments were performed at different mole ratios and fixed temperature. Two types of zeolitic catalysts were used for these experiments: the HY zeolite and zeolite Beta. Here are some pictures of their structure obtained from the Database of Zeolite Structures:
| structure of HY zeolite | structure of zeolite Beta |
 |
 |
The channels of HY zeolite are composed of 12 Oxygen atoms forming a ring of 7.4 Å:
On the other hand, zeolite Beta is composed of two types of channels along different axis:
|
Channels viewed along axis 001
|
Channels viewed along axis 100
|
As previously observed with other catalysts this reaction is thermodynamically limited after 85°C. Therefore to work in the kinetic regime the experiments at varying feed mole ratios were performed at 75°C. Langmuir-Hinshelwood rate expressions were used to try to analyse these experiments. The reactor model considered only a mass balance without any diffusion limitations. These computations showed a good correspondence between the model and the experiments on zeolite Beta. However with the aid of the Langmuir-Hinshelwood rate expressions it is not possible to interpret the experiments with the HY zeolite. Further calculations showed that external diffusion limitations were minimal and that internal diffusion in the catalyst pores was significant.
Also investigations on working catalyst using the in situ steady state isotope transient kinetic analysis (SSITKA) should give more information on the diffusion of the reactants, the catalyst surface reaction. This apparatus was developped and managed by Dr. James A. Sullivan and supervised by Prof. Robbie Burch. At the time of this project, Prof. Burch was directing the Catalysis Research Centre in the University of Reading.
![]()