 |
||||
|
|
||||
 |
||||
Research - Chemical Strategies |
||||
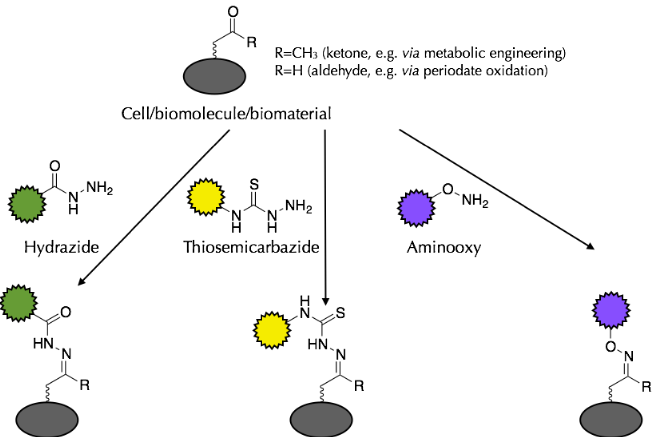
Biomaterials can be chemically modified with a wide range of molecules to improve and tailor their properties for a number of biomedical applications, including controlled drug delivery, targeted drug delivery, in vivo imaging, tissue engineering and regenerative medicine. It is even possible to chemically modify the surfaces of living cells, both in vitro and in vivo, to elicit a desired response. We have a particular interest in applying chemical strategies to improve cellular interactions with biomaterials and to address limitations in current tissue engineering approaches such as cell patterning and the guidance of cell growth in 3D. However, carrying out chemical reactions in the presence of cells, tissue and many biomaterials can be problematical as they possess chemical groups that could undergo non-specific side reactions, potentially resulting in a negative impact on their function. To overcome this, we employ chemoselective ligation reactions, which rely on mutually-reactive molecules with chemical functionalities not normally found within biological systems. This means that these species cannot react non-specifically with biomaterials or molecules found in living tissues, removing the possibility of unwanted and possibly detrimental side reactions. One particularly useful chemoselective ligation reaction is that of carbonyl groups (aldehydes or ketones) with hydrazide-, thiosemicarbazide- or aminooxy-containing molecules. Carbonyls can be readily and specifically incorporated into biomaterials and living cells and can then be utilized as “chemical handles” for subsequent chemoselective decoration: |
||
 |
||
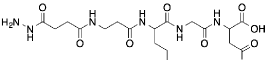
We have utilized this approach for the following applications: Biomaterial Modification |
||
 |
||||||
Conversely, we have also arrested cell growth, specifically that of motoneurons, by the modification of surfaces with a “stop” signal (Lue-Arg-Glu hydrazide). |
||||||
RGD hydrazide |
||||||
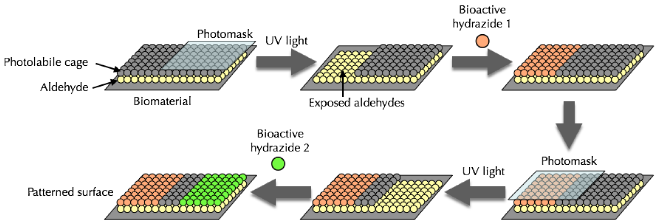
Formation of 3D Cell Aggregates Caged Aldehydes |
||
 |
||
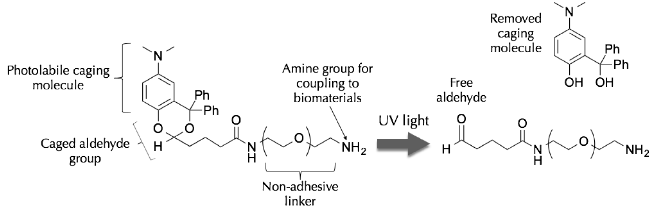
Ideally, this approach could utilize a wide range of biomaterials, offer rapid removal of the photolabile cage and render the surface non-adhesive, except where an adhesive ligand has been chemoselectively attached. To fulfill these criteria, we designed the following molecule: |
||
 |
||
For more information, please follow this link. |
||