|
Enantioselective
occurrence and fate of chiral drugs in the environment |
Funder: Natural Environment Research Council Project No: NE/I000534/1 (project details) |
|||||
|
Chirality plays an important role in the life of plants and
animals but it is also vital in the agricultural, pharmaceutical and chemical
industries. The phenomenon of chirality is also of growing importance in the
field of environmental pollution and its effects on human health. More than
half of the pharmacologically active compounds (PACs) currently in use are
chiral compounds and many of those are marketed as racemates consisting of an
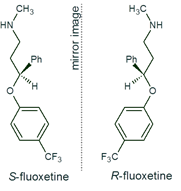
equimolar mixture of two enantiomers (1). A chiral molecule usually has at least one chiral centre (e.g.
asymmetric carbon) as a result of which it shows optical activity. It exists
in the form of two enantiomers (if only one chiral centre is present), being
the non-superimposable mirror images of each other. Enantiomers of the same
chiral molecule have similar physico-chemical properties but may differ in
their biological properties. Distribution, metabolism and excretion usually
favour one enantiomer over the other. This results from the fact that
enantiomers stereoselectively react in biological systems, e.g. with enzymes.
Additionally, due to different activity, chiral molecules can differ in
toxicity. Thalidomide is an
excellent example. A therapeutic (+)-thalidomide is harmless but in
the human body it undergoes in vivo inter-conversion leading to toxic (-)-enantiomer, which leads to
malformations of embryos if administered to pregnant woman (1). Therefore, the enantiomeric composition of a chiral molecule can
change throughout its environmental life-cycle. It can be altered after its
administration as a result of its metabolism in the body. Enantiomeric
composition of the chiral molecule can be subsequently changed during
wastewater treatment and in the environment. Therefore the very same chiral
molecule might have different activity/toxicity at different stages of its
environmental life cycle, which will depend on its origin and exposure to
environmental factors. |
||||||
|
CHIRAL PACs IN THE ENVIRONMENT AND THEIR
UNDISCOVERED ENANTIOMER DEPENDANT FATE |
PACs are emerging environmental contaminants. Thousands of PACs
are approved for human/veterinary use, although only a very small percentage
of these compounds have been studied in the environment. Some of the most
commonly used PACs are sold in hundreds of tonnes/year in the UK alone. Usage
of PACs is likely to increase in the future due to an ageing population in
western countries and an increase in consumption levels in the developing
world. PACs enter the environment mainly through insufficiently treated
sewage, waste effluents from manufacturing processes, runoff and sludge. They
are ubiquitous and persistent with synergistic properties. PACs have also
been detected in drinking water, which poses a direct risk to humans. Surprisingly, the
environmental fate and toxicity of PACs are assessed without taking into
consideration their enantiomeric forms. This might lead to a significant
under or overestimation of toxicity of chiral PACs and to incorrect
environmental risk assessment as chiral PACs are likely to be present in the
environment in their non-racemic forms.
References: (1) B.
Kasprzyk-Hordern, Chemical Society Reviews 39 (2010) 4466. (2) Stanley, J.K.,
Ramirez, A.J., Chambliss, C.K., Brooks, B.W., Chemosphere 69 (2007) 9-16. (3) S.L. MacLeod,
P. Sudhir, C.S. Wong. Journal of Chromatography 1170 (2007) 23 |
|||||
|
This project aims
to identify chiral drugs in the aqueous environment and to test the
hypothesis that their distribution in the aqueous environment is
stereoselective and that stereoselective mechanisms governing their fate are
biological in nature. The project will
be undertaken taking into account the following objectives: Objective 1: To
establish and validate multi-residue analytical methods for the
quantification of chiral drugs using SPE-chiral-LCMS/MS instrumentation. Objective 2: To
analyse enantiomers of chiral drugs and their metabolites in the aqueous
environment and to test the hypothesis that distribution of chiral drugs in
the aqueous environment is stereoselective. Objective 3: To
verify stereoselectivity in degradation pathways of chiral drugs in surface
water in microcosm experiments in order to test the hypothesis that
stereoselective mechanisms governing their fate are biological in nature. |
||||||
|
1.
Development of novel methodology for enantiomeric profiling of
chiral drugs in aqueous environmental matrices. A novel multi-residue methodology for enantiomeric profiling of
chiral drugs in different environmental matrices utilising for the first time
high resolution QTOF MS was developed. This method allows for both target
analysis and screening of unknowns and is of key importance if mechanisms of
degradation are studied. 2.
Verification of enantiomer-specific fate of chiral drugs in the
UK aqueous environment. Enantiomeric profiling of chiral drugs of abuse in the
environment has never been a subject of investigation before. It revealed the
enantiomer-specific fate of all studied drugs. The extent of
stereoselectivity depended on several parameters including: type of a chiral
drug, wastewater treatment technology used and season. 3.
Discovery of enantiomer-specific biotransformation of chiral
drugs in river microcosms. Laboratory microcosm experiments undertaken for drugs of abuse
proved, in the first ever study of this kind, the hypothesis that
stereoselective mechanisms governing fate of chiral drugs of abuse are
biological in nature. This ground-breaking project proved for
the first time that chiral drugs of abuse are subject to enantiomer-specific
processes occurring in the environment and that the enantiomeric composition of a chiral drug can change
throughout its environmental cycle. Knowing that two enantiomers of the same
chiral drug usually differ in potency and toxicity (e.g. S(+)-amphetamine has twice as high
stimulant activity than R(−)-amphetamine), the very same chiral compound might have different
activity/toxicity at different stages of its environmental life cycle, which
will depend on its origin and exposure to environmental factors. The above is
of critical significance in the environmental risk assessment of
pharmacologically active compounds, which currently does not take into
account enantiomerism of pollutants and potentially leads to a significant
under or overestimation of toxicity of chiral drugs. |
||||||
|
|
Publications: Evans, S. E., Davies, P., Lubben, A.
and Kasprzyk-Hordern, B., 2015. Forthcoming. Determination of chiral
pharmaceuticals and illicit drugs in wastewater and sludge using microwave
assisted extraction, solid-phase extraction and chiral liquid chromatography
coupled with tandem mass spectrometry. Analytica Chimica Acta, 882, pp.
112-126. Camacho-Muñoz,
D., Petrie, B., Castrignano, E. and Kasprzyk-Hordern, B., 2015. Enantiomeric
Profiling of Chiral Pharmacologically Active Compounds in the Environment
with the usage of chiral Liquid Chromatography Coupled with Tandem Mass
Spectrometry. Current Analytical Chemistry, 12. Petrie, B., Camacho-Munoz, M., Castrignano, E., Evans, S. and
Kasprzyk-Hordern, B., 2015. Chiral Liquid Chromatography Coupled with Tandem
Mass Spectrometry for Environmental Analysis of Pharmacologically Active
Compounds. LCGC Europe, 2 (28), p. 151. Evans, S. and Kasprzyk-Hordern, B.,
2014. Applications of chiral chromatography coupled with mass spectrometry in
the analysis of chiral pharmaceuticals in the environment. Trends in
Environmental Analytical Chemistry, 1 (1), 1. Bagnall, J., Malia, L., Lubben, A. and
Kasprzyk-Hordern, B., 2013. Forthcoming. Stereoselective biodegradation of
amphetamine and methamphetamine in river microcosms. Water Research, 47 (15),
pp. 5708-5718.. Bagnall, J.P., Evans, S.E., Wort,
M.T., Lubben, A.T. and Kasprzyk-Hordern, B., 2012. Using chiral liquid
chromatography quadrupole time-of-flight mass spectrometry for the analysis
of pharmaceuticals and illicit drugs in surface and wastewater at the
enantiomeric level. Journal of Chromatography A, 1249, pp. 115-129. Kasprzyk-Hordern, B. and Baker, D. R.,
2012. Estimation of community-wide drugs use via stereoselective profiling of
sewage. Science of the Total Environment, 423, pp. 142-150. Kasprzyk-Hordern, B. and Baker, D. R., 2012. Enantiomeric
profiling of chiral drugs in wastewater and receiving waters. Environmental
Science & Technology, 46 (3), pp. 1681-1691. Presentations: Kasprzyk-Hordern,
B., 2015. Stereochemistry of Pharmacologically Active Compounds:A New
Paradigm in Environmental Analysis and Risk Assessment. In: ‘Emerging
contaminants in waters and soils, practical considerations: -Sampling,
analysis and consequences’, Royal Society of Chemistry Water Science Forum,
2015-03-04 - 2015-03-04, Sheffield. Kasprzyk-Hordern,
B., 2015. Urban water profiling for community-wide public health assessment.
In: IX Polish Conference on Analytical Chemistry, 2015-07-06 - 2015-07-09,
Poznan. Kasprzyk-Hordern,
B., Castrignano, E., Rydevik, A., Lopardo, L., Rice, J. and Yang, Z., 2015.
Wastewater-based epidemiology and future perspectives:testing urban water for
community-wide public health assessment. In: 15th EuCheMS International
Conference on Chemistry and the Environment, 2015-09-20 - 2015-09-25,
Leipzig. Kasprzyk-Hordern,
B., ‘Divining human health through urban wastewater profiling’, Bath Science
Café, Bath, 2014-09-08 Kasprzyk-Hordern,
B., ‘Testing urban water for community-wide public health assessment’, CCAF
Symposium ‘The alchemy of what we swallow’, 2014-09-09, Bath Kasprzyk-Hordern,
B., ‘Chiral chromatography coupled with tandem mass spectrometry for
enantiomeric profiling of chiral pharmacologically active compounds’, 9th
Aegean Analytical Chemistry Days, Chios, Greece, 2014 Kasprzyk-Hordern,
B., 2014. Urban water cycle: environmental and health perspectives, STEM CPD
Day, 2014-06-18, Bath. Kasprzyk-Hordern,
B., 2014. Water and you: green, grey and blue’, Pint-of-Science Festival,
2014 -05-19, Bath. Kasprzyk-Hordern,
B., 2014. Testing wastewater in public health epidemiology: Pharmaceuticals,
illicit drugs and the phenomenon of chirality. In: The SfAM Meeting
"Control of water-borne disease: A century of the activated sludge
sewage treatment process", 2014-04-01 - 2014-04-02, Manchester. Kasprzyk-Hordern,
B., 2013. Wastewater profiling at a community level: a new paradigm in
epidemiological studies of public health, University of Bristol, School of
Social and Community Medicine, 2013-0-01, Bristol Evans, S., Bagnall, J. and Kasprzyk-Hordern, B., 2013. Chiral
illicit drugs and pharmaceuticals – stereoselective degradation, and its implications
for analysis, toxicology and regulatory frameworks. In: Joint Annual Meeting of the Ecotoxicology Research and Innovation
Centre Plymouth University, and the Society of Environmental Toxicology and
Chemistry UK Branch,
2013-09-09 - 2013-09-10, Plymouth. Kasprzyk-Hordern,
B., 2013. Illicit drugs in wastewater: chirality
and other under-investigated phenomena. In:
14th EuCheMS International Conference on Chemistry and the Environment,
Satellite Event 'Illicit drugs in wastewater', 2013-06-25, Barcelona. Kasprzyk-Hordern,
B., Bagnall,
J., Baker, D. and Evans,
S., 2013. Enantiomerism of medicinal products – a
new paradigm in environmental risk assessment. In: Book of abstracts, 23rd Annual Meeting of
the Society of Environmental Toxicology and Chemistry (SETAC Europe),
2013-05-12 - 2013-05-16, Glasgow. Kasprzyk-Hordern,
B., 2013. Wastewater
analysis — an emerging science: key issues and their application: General
overview of analytical methods and chiral analysis. In: Testing the
waters: first international multidisciplinary conference on detecting illicit
drugs in wastewater, 2013-05-06 - 2013-05-08, Lisbon. Kasprzyk-Hordern,
B., 2013. Enantioselective
analysis of chiral pharmacologically active compounds in urban water. In: The RSC
Analytical Division Separation Science Group and the RSC Environmental
Chemistry Group Meeting, 'Recent Advances in the Analysis of Complex
Environmental Matrices', 2013-02-28, London. B. Kasprzyk-Hordern, ‘The significance of chirality of illicit
drugs for the estimation of drugs of abuse using the sewage epidemiology
approach’ EMCDDA
Workshop ‘The determination of illicit drug consumption in populations
through wastewater biomarker analysis’, Lisbon, Portugal, 2012. Kasprzyk-Hordern, B., 2012. Enantiomeric profiling of chiral drugs in
the environment with the usage of Chiral-LCMS/MS. In: 8th Annual LC/MS/MS Workshop on Environmental Applications
and Food Safety, 2012-07-01 - 2012-07-03, Barcelona. Bagnall, J., Malia, L., Lubben, A. and Kasprzyk-Hordern, B.,
2012. The stereo-selective
biodegradation of amphetamine and methamphetamine in river water using
chiral-LC-QTOFMS. In:
8th Annual LC/MS/MS Workshop on Environmental Applications and Food Safety,
2012-07-01 - 2012-07-03, Barcelona. Kasprzyk-Hordern, B. and Baker, D. R., 2012. PACs during wastewater treatment and in
receiving waters – emerging issues. In: 6th SETAC World Congress/SETAC Europe 22nd Annual Meeting,
2012-05-19 - 2012-05-23, Berlin. Kasprzyk-Hordern, B. and Baker, D., 2011. The significance of chirality of illicit
drugs for the estimation of drugs abuse using the sewage epidemiology
approach. In: SETAC Europe 21st Annual Meeting, 2011-05-14 - 2011-05-18,
Milan. |
|||||
|
|
||||||
Dr Barbara Kasprzyk-Hordern, University
of Bath, Department of Chemistry, Bath BA2 &AY, UK, mailto:B.Kasprzyk-Hordern@bath.ac.uk