University Press Releases:
 |
| Artistic representation of hyper-Raman optical activity. Credit: Ventsislav Valev and Kylian Valev |
|
Physicists use light to probe deeper into the ‘invisible’ energy states of molecules
31 July 2024 University
of Bath
A team led by scientists at the University of Bath discovers how light particles can be used to reveal the ‘hidden’ energy states of molecules.
A new optical phenomenon has been demonstrated by an international team of scientists led by physicists at the University of Bath, with significant potential impact in pharmaceutical science, security, forensics, environmental science, art conservation and medicine.
Molecules rotate and vibrate in very specific ways. When light shines on them it bounces and scatters. For every million light particles (photons), a single one changes colour. This change is the Raman effect. Collecting many of these colour-changing photons paints a picture of the energy states of molecules and identifies them.
Yet some molecular features (energy states) are invisible to the Raman effect. To reveal them and paint a more complete picture, ‘hyper-Raman’ is needed.
Hyper-Raman
The hyper-Raman effect is a more advanced phenomenon than simple Raman. It occurs when two photons impact the molecule simultaneously and then combine to create a single scattered photon that exhibits a Raman colour change.
Hyper-Raman can penetrate deeper into living tissue, it is less likely to damage molecules and it yields images with better contrast (less noise from autofluorescence). Importantly, while the hyper-Raman photons are even fewer than those in the case of Raman, their number can be greatly increased by the presence of tiny metal pieces (nanoparticles) close to the molecule.
Despite its significant advantages, so far hyper-Raman has not been able to study a key enabling property of life – chirality.
Optical activity
In molecules, chirality refers to their sense of twist – in many ways similar to the helical structure of DNA. Many bio-molecules exhibit chirality, including proteins, RNA, sugars, amino acids, some vitamins, some steroids and several alkaloids.
Light too can be chiral and in 1979, the researchers David L. Andrews and Thiruiappah Thirunamachandran theorised that chiral light used for the hyper-Raman effect could deliver three-dimensional information about the molecules, to reveal their chirality.
However, this new effect – known as hyper-Raman optical activity – was expected to be very subtle, perhaps even impossible to measure. Experimentalists who failed to observe it struggled with the purity of their chiral light. Moreover, as the effect is very subtle, they tried using large laser powers, but this ended up damaging the molecules being studied.
Explaining, Professor Ventsislav Valev who led both the Bath team and the study, said: “While previous attempts aimed to measure the effect directly from chiral molecules, we took an indirect approach.
“We employed molecules that are not chiral by themselves, but we made them chiral by assembling them on a chiral scaffold. Specifically, we deposited molecules on tiny gold nanohelices that effectively conferred their twist (chirality) to the molecules.
“The gold nanohelices have another very significant benefit – they serve as tiny antennas and focus light onto the molecules. This process augments the hyper-Raman signal and helped us to detect it.
“Such nanohelices were not featured on the 1979 theory paper and in order to account for them we turned to none other than one of the original authors and pioneer of this research field.”
Confirming a 45-year-old theory
Emeritus Professor Andrews from the University of East Anglia and co-author of the paper said: “It is very gratifying to see this work the experimental finally confirm our theoretical prediction, after all these years. The team from Bath have performed an outstanding experiment.”
This new effect could serve to analyse the composition of pharmaceuticals and to control their quality. It can help identify the authenticity of products and reveal fakes. It could also serve to identify illegal drugs and explosives at customs or crime scenes.
It will aid detecting pollutants in environmental samples from air, water and soil. It could reveal the composition of pigments in art for conservation and restoration purposes, and it will likely find clinical applications for medical diagnosis by detecting disease-induced molecular changes.
Professor Valev said: “This research work has been a collaboration between chemical theory and experimental physics across many decades and across academics of all stages – from PhD student to Emeritus Professor.
“We hope it will inspire other scientists and that it will raise awareness that scientific progress often takes many decades.”
Looking ahead he added: “Ours is the very first observation of a fundamental physical mechanism. There is a long way ahead until the effect can be implemented as a standard analytical tool that other scientist can adopt.
“We look forward to the journey, together with our collaborators from Renishaw PLC, a world-renowned manufacturer of Raman spectrometers.”
Dr Robin Jones, first-author for the new research paper and a PhD student at Bath until recently, said: “Performing the experiments that showed the hyper-Raman optical activity effect has been my most rewarding academic experience. In retrospect, it seems that almost every step of my PhD was like a piece of the puzzle which fell into place to achieve the observation.”
The research is published in the journal Nature Photonics. It was funded by The Royal Society, the Leverhulme Trust, and the Engineering and Physical Science Research Council (EPSRC).
|
 |
| Blue eyes owe their colour to the Tyndall effect. |
|
The nanotechnological revolution requires standardised ‘screws’ – here is a way to measure them
17 June 2024 University
of Bath
Physicists at the University of Bath lead on the discovery of a new optical property that measures the twist in tiny helices.
A new nonlinear optical property of tiny particles has been discovered by an international team of scientists led by physicists at the University of Bath, with important implications for researchers working in fields as diverse as display technology, chemical catalysis and medicine.
The new property is seen when light passing through tiny particles – similar in size to the wavelength of light – is scattered at a colour that differs from that of illumination. The scattered light is at the ‘second-harmonic frequency’, meaning it’s at twice the frequency of the illuminating light.
The study set out to explore the Tyndall effect – the phenomenon of light scattering from particles that are larger than nanoparticles but smaller than microparticles. Particles of this size include viruses and single cell organisms, such as bacteria.
When illuminated with white light, such particles appear blue (blue eyes also owe their colour to the Tyndall effect).
Second-harmonic Tyndall scattering
Inorganic particles dispersed in liquids are useful in many applications, including the adding of colour to paints and plastics, UV protection creams (zinc oxide and titanium dioxide scatter ultraviolet light but let visible light through), catalysis (to speed up or enable chemical reactions), and medical therapeutics (examples include encapsulating drugs and delivering them to their target; selectively cutting DNA, and killing viruses).
For all these applications, it’s essential for researchers to characterise the particles’ size and shape, accurately and in real-time.
Light is the best method to perform such analysis on particles in water, which is often the medium they are held in. When particles are illuminated, their scattered light holds information about both their size and geometry.
Several methods for analysing particle size depend on the Tyndall effect. Most methods rely on weak light sources (typically lamps) and the collected scattered light is of the same colour as the illumination. Other, more sophisticated methods rely on a laser light source. The new study takes scientists’ understanding of light scattered by laser to the next level.
Explaining, Professor Ventsislav Valev, who led both the Bath team and the study, said: “When a laser – with long light wave – is used in Tyndall’s experiment, light can be created at a different colour – with short wave – and then scattered. The new colour corresponds to twice the light vibration of illumination.
“This discovery was made in 1965 in the laboratories of Ford Motor Company and applies to particles of all sizes. But if a particle’s size matches the Tyndall effect range, then the illuminating and the newly created light can be better separated in space. Basically, the Tyndall effect sorts light waves by size.
He added: “But one geometrical property has remained unobservable until now with this new study: chirality!”
Twisted molecules
Chirality is a fundamental geometrical property across practically all length scales. In humans and other living organisms, all the functional amino acids are chiral, and so are sugars, proteins, and so on. Chirality is expressed in the direction of a molecule’s twist (clockwise or anticlockwise), akin to the twist of a DNA helix.
For the new study, team members from the United States fabricated silicon helices with length of about 270 nm, which corresponds in size to some viruses, large exosomes and bacteriophages.
Professor Valev said: “We discovered that when we illuminate these helices with chiral (or circularly polarised) laser light, the scattered light can tell us which way silicon helices wind up.
“One reason this is important is because silicon is the most abundant solid element on Earth, so every new property holds potential for sustainable and cost-effective applications.
“Another reason is that measuring twist (chirality) is much needed for assembling inorganic materials from nanotechnological building blocks. The importance is similar to that of making and then being able to measure the thread of a standardised screw.”
Looking ahead, Professor Valev said: “Now that we have a baseline for the properties of single helices in water, the next stage is to start modifying them and eventually building them into self-assembled materials.”
PhD student Ben Olohan, first-author on the research publication, said: “The key here is that biological processes extend from molecules to cell assemblies and beyond. Compared to the length scales of Tyndall scattering, similar effects have been observed for much smaller and for much larger particles.
“So, this intermediate length scale effect had to exist, yet remained unobserved. This is why I kept looking hard for its demonstration. It feels very satisfying for my PhD project, to have found such a ‘missing link’ in science.”
The research is published in the journal ACS Nano. It was funded by The Royal Society, the Leverhulme Trust, and the Engineering and Physical Science Research Council (EPSRC).
|
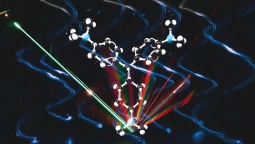 |
| Crystal violet scatters light into a rainbow, revealing the strength of interaction between light and helical nanostructures (artist's impression by photography). Image credit: Ventsislav Valev, Kylian Valev, Eva Valev, Robin Jones |
|
Can rainbows monitor the environment?
3 August 2023 University
of Bath
New nanotechnology may make it easier to identify the chemical composition of impurities and their geometrical shape in samples of air, liquid and live tissue.
Using conventional testing techniques, it can be challenging – sometimes impossible – to detect harmful contaminants such as nano-plastics, air pollutants and microbes in living organisms and natural materials. These contaminants are sometimes found in such tiny quantities that tests are unable to reliably pick them up.
This may soon change, however. Emerging nanotechnology (based on a ‘twisted’ state of light) promises to make it easier to identify the chemical composition of impurities and their geometrical shape in samples of air, liquid and live tissue. An international team of scientists led by physicists at the University of Bath is contributing towards this technology, which may pave the way to new environmental monitoring methods and advanced medicines.
The emerging chemical-detection technique is based on a light-matter interaction known as the Raman effect. The Raman effect occurs when a material that is illuminated at a certain colour of light scatters and changes the light into a multitude of slightly different colours. It essentially produces a mini-rainbow that is dependent on how atoms within materials vibrate.
Measuring the colours of the Raman rainbow reveals individual atomic bonds because molecular bonds have distinct vibrational patterns. Each bond within a material produces its own unique colour change from that of the illumination. Altogether, the colours in the Raman rainbow serve to detect, analyse and monitor the chemical composition (chemical bonds) of complex molecules, such as those found within mixtures of environmental pollutants.
“The Raman effect serves to detect pesticides, pharmaceuticals, antibiotics, heavy metals, pathogens and bacteria. It’s also used for analysing individual atmospheric aerosols that impact human health and the climate,” said Dr Robin Jones from the Department of Physics at Bath, who is the first-author of the study.
The study is published in the journal Advanced Materials.
Harmful pollutants
Expanding, co-author Professor Liwu Zhang from the Department of Environmental Science at Fudan University in China said: “Aquatic pollutants, even in trace amounts, can accumulate in living organisms through the biological chain. This poses a threat to human health, animal welfare and wildlife. Generally, it is really hard to know exactly what the chemical composition of complex mixtures are.”
Professor Ventsislav Valev from Bath, who led the study, added: “Understanding complex, potentially harmful pollutants in the environment is necessary, so that we can learn how to break them down into harmless components. But it is not all about what atoms they are made of. The way the atoms are arranged matters a lot – it can be decisive for how molecules act, especially within living organisms.
“Our work aims to develop new ways in which the Raman effect can tell us about the way atoms are arranged in space and now we have taken an important technological step using tiny helix shaped antennas made of gold.”
The Raman effect is very weak – only 1 out of 1,000,000 photons (light particles) undergo the colour change. In order to enhance it, scientists use miniature antennas fabricated at the nanoscale that channel the incident light into the molecules. Often these antennas are made of precious metals and their design is limited by nanofabrication capabilities.
The team at Bath used the smallest helical antennas ever employed: their length is 700 times smaller than the thickness of a human hair and the width of the antennas is 2,800 times smaller. These antennas were made from gold by scientists in the team of Professor Peer Fischer at the University of Stuttgart in Germany.
“Our measurements show these helical antennas help to get a lot of Raman rainbow photons out of molecules,” said Dr Jones. “But more importantly, the helical shape enhances the difference between two types of light that are often used to probe the geometry of molecules. These are known as circularly polarised light.
“Circularly polarised light can be left-handed or right-handed and our helices can, basically, handshake with light. And because we can make the helices twist to the left or to the right, the handshake with light that we devised can be both with left or right ‘hands’.
“While such handshakes have been observed before, the key advance here is that we demonstrate for the first time that it is felt by molecules, as it affects their Raman rainbow. This is an important step that will allow us to distinguish efficiently and reliably between left- and right-handed molecules, first in the lab and then in the environment.”
Crystal Violet
In order to demonstrate that the new handshaking between light and antennas could be transmitted to molecules, the researchers made use of molecules – crystal violet – that are unable to ‘handshake’ with light by themselves. Yet these molecules behaved as if they could perform this function, expressing the ‘handshaking’ ability of gold nanohelices to which they were attached.
“Another important aspect of our work here is that we worked with two industrial partners,” said Professor Valev. “VSPARTICLE produce standard nanomaterials for measuring Raman light. Having common standards is really important for researchers around the world to be able to compare results.”
He added: “Our industrial partner Renishaw PLC is a world-leading manufacturer of Raman spectroscopy and microscopy equipment. Such partnerships are essential, so that new technology can move out of the labs and into the real-world, where the environmental challenges are.”
Building on this work, the team is now working on developing more advanced forms of Raman technologies.
The research team from the University of Bath included Dr Robin Jones, Dr Kristina Rusimova, Professor Daniel Wolverson and Professor Ventsislav Valev.
|
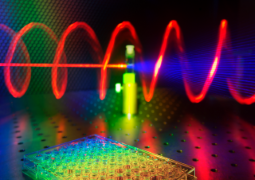 |
| Upon illuminating chiral semiconductor nanoparticles with circularly polarised light (in red), third harmonic Mie scattering light streams out (in blue). Credit: Ventsislav Valev, Kylian Valev and Lukas Ohnoutek. |
|
Keeping up with the first law of robotics: A new photonic effect for accelerated drug discovery
13 January 2022 University
of Bath
Physicists at the University of Bath and University of Michigan demonstrate a new photonic effect in semiconducting nanohelices.
A new photonic effect in semiconducting helical particles with nanoscale dimensions has been discovered by an international team of scientists led by researchers at the University of Bath. The observed effect has the potential to accelerate the discovery and development of life-saving medicines and photonic technologies.
In his Robot series, science-fiction writer Isaac Asimov imagined a future where robots grew into trustworthy companions for humans. These robots were guided by the laws of robotics, the first of which states that 'a robot may not injure a human being or, through inaction, allow a human being to come to harm'. Thanks to the new photonic discovery, robots may get a chance to prevent humans from coming to harm in a very meaningful way – by greatly speeding up the development of important drugs, such as new antibiotics.
Currently, the World Health Organisation regards antibiotic resistance (the growing ineffectiveness of drugs currently on the market) as one of the top 10 threats to humanity. Moreover, globalisation coupled with human encroachment into wildlife habitats increases the risk of new infectious diseases emerging. It is widely recognised that the cost of discovering and development new drugs for these and other conditions using today’s technology is unsustainable. The need for pharmaceutical research to be accelerated has never been more pressing and it would benefit hugely from the help of artificial intelligence (AI).
Bath Physics professor Ventsislav Valev, who headed the research, said: “Although we are a long way still from Asimov’s positronic robot brains, our latest finding does have the potential to link AI algorithms that analyse chemical reactions and robotic arms that prepare chemical mixtures – a process known as high-throughput screening.”
Meeting the needs of robotised chemistry
High-throughput screening (HTS) is an experimental method that uses robots to discover new drugs. Some labs have adopted it already, to help them analyse vast libraries of molecules. In the future, however, discovering new drugs could happen entirely through HTS. Using this method, robots simultaneously operate a large number of syringes, preparing thousands of chemical mixtures that are then robotically analysed. The results are fed back to AI algorithms, which then determine what mixtures to prepare next, and so on until a useful drug is discovered.
The analytical step is key, since without it, the robots cannot know what they have prepared.
HTS happens on microplates (or tablets) that are about the size of a chocolate bar. Each tablet contains wells into which the chemical mixtures are poured. The more wells found on a tablet, the more chemicals can be analysed in one hit. But though a modern tablet can host thousands of wells, the size of the table does not change.
“To meet the requirements of the emerging robotised chemistry, wells are getting really tiny – too small for current analytical methods,” said Professor Valev. “So, fundamentally new methods are needed to analyse would-be drugs.
“Currently, most new drugs that are entering the market and the majority of old drugs are chiral (their chemical formula lacks mirror symmetry). Therefore it is especially important to be able to measure chirality in tiny volumes of less than 1 mm3 which is about the size of a cube with sides of the thickness of a credit card.”
The effect discovered by the researchers allows chirality to be measured in volumes that are 10,000 times smaller than 1 mm3.
“We have used a very exciting new material developed by our colleagues at the University of Michigan in the US, led by Professor Nicholas Kotov,” explained Professor Valev. “It’s a biomimetic structure (i.e. one that simulates biological phenomena) that chemically assembles into semiconducting helices, at the nanoscale, similarly to the way proteins assemble.”
Professor Kotov said: “Being illuminated with red light, the small semiconductor helices generate new light that is blue and twisted. The blue light is also emitted in a specific direction, which makes it easy to collect and analyse. The trifecta of unusual optical effects drastically reduce the noise that other nanoscale molecules and particles in biological fluids may cause.”
Professor Valev added: “This means that by carefully measuring the blue light, we can ascertain the direction of twist (or chirality) of the structures we’re studying.”
The twist of the nanohelices can change dramatically depending on the kind of biomolecules that were present when these helixes formed, providing a wealth of information about the biological samples.
“Our results open the way for measuring chirality in volumes potentially 10-million times smaller than 1 mm3. Although the structures that we measured so far are much larger than typical pharmaceuticals, we have proven that the physical effect is real, so in principle, applications to molecules and especially drugs are now only a question of technological development. Our next step is to seek funding for this development,” said Professor Valev.
PhD student Lukas Ohnoutek, also involved in the research, said: “In nanotechnology, one of the big challenges is to be able to see the properties of tiny things. Nowadays, this is easy for stationary objects but it’s still hard for an object that freely floats in a liquid.
“It has been extremely gratifying to reduce our volume of study so successfully – we now focus light to a spot that would be invisible to most people’s eyes. And within that volume, we can determine the direction of twist of helices that are much smaller still.”
The research is published in the journal Nature Photonics. It was funded by The Royal Society, the Science and Technology Facilities Council (STFC) and the Engineering and Physical Science Research Council (EPSRC). Read full paper. |
 |
| Upon illumination with red light, third harmonic scattered light (in violet) reveals the twist of metal nanoparticles. (Credit: Ventsislav Valev & Lukas Ohnoutek). |
|
The nanophotonics orchestra presents: Twisting to the light of nanoparticles
23 September 2021 University
of Bath
Physicists at the University of Bath observe a new physical effect in chiral (twisted) nanoparticles.
Physics researchers at the University of Bath discover a new physical effect relating to the interactions between light and twisted materials – an effect that is likely to have implications for emerging new nanotechnologies in communications, nanorobotics and ultra-thin optical components.
In the 17th and 18th centuries, the Italian master craftsman Antonio Stradivari produced musical instruments of legendary quality, and most famous are his (so-called) Stradivarius violins. What makes the musical output of these musical instruments both beautiful and unique is their particular timbre, also known as tone colour or tone quality. All instruments have a timbre – when a musical note (sound with frequency fs) is played, the instrument creates harmonics (frequencies that are an integer multiple of the initial frequency, i.e. 2fs, 3fs, 4fs, 5fs, 6fs, etc.).
Similarly, when light of a certain colour (with frequency fc) shines on materials, these materials can produce harmonics (light frequencies 2fc, 3fc, 4fc, 5fc, 6fc, etc.). The harmonics of light reveal intricate material properties that find applications in medical imaging, communications and laser technology.
For instance, virtually every green laser pointer is in fact an infrared laser pointer whose light is invisible to human eyes. The green light that we see is actually the second harmonic (2fc) of the infrared laser pointer and it is produced by a special crystal inside the pointer.
In both musical instruments and shiny materials, some frequencies are ‘forbidden’ – that is, they cannot be heard or seen because the instrument or material actively cancels them. Because the clarinet has a straight, cylindrical shape, it supresses all of the even harmonics (2fs, 4fs, 6fs, etc.) and produces only odd harmonics (3fs, 5fs, 7fs, etc.). By contrast, a saxophone has a conical and curved shape which allows all harmonics and results in a richer, smoother sound. Somewhat similarly, when a specific type of light (circularly polarised) shines on metal nanoparticles dispersed in a liquid, the odd harmonics of light cannot propagate along the direction of light travel and the corresponding colours are forbidden.
Now, an international team of scientists led by researchers from the Department of Physics at the University of Bath have found a way to reveal the forbidden colours, amounting to the discovery of a new physical effect. To achieve this result, they ‘curved’ their experimental equipment.
Professor Ventsislav Valev, who led the research, said: “The idea that the twist of nanoparticles or molecules could be revealed through even harmonics of light was first formulated over 42 years ago, by a young PhD student – David Andrews. David thought his theory was too elusive to ever be validated experimentally but, two years ago, we demonstrated this phenomenon. Now, we discovered that the twist of nanoparticles can be observed in the odd harmonics of light as well. It’s especially gratifying that the relevant theory was provided by none other than our co-author and nowadays well-established professor – David Andrews!
“To take a musical analogy, until now, scientists who study twisted molecules (DNA, amino acids, proteins, sugars, etc) and nanoparticles in water – the element of life – have illuminated them at a given frequency and have either observed that same frequency or its noise (inharmonic partial overtones). Our study opens up the study of the harmonic signatures of these twisted molecules. So, we can appreciate their ‘timbre’ for the first time.
“From a practical point of view, our results offer a straightforward, user-friendly experimental method to achieve an unprecedented understanding of the interactions between light and twisted materials. Such interactions are at the heart of emerging new nanotechnologies in communications, nanorobotics and ultra-thin optical components. For instance, the ‘twist’ of nanoparticles can determine the value of information bits (for left-handed or right-handed twist). It is also present in the propellers for nanorobots and can affect the direction of propagation for a laser beam. Moreover, our method is applicable in tiny volumes of illumination, suitable for the analysis of natural chemical products that are promising for new pharmaceuticals but where the available material is often scarce.
PhD student Lukas Ohnoutek, also involved in the research, said: “We came very close to missing this discovery. Our initial equipment was not ‘tuned’ well and so we kept seeing nothing at the third-harmonic. I was starting to lose hope but we had a meeting, identified potential issues and investigated them systematically until we discovered the problem. It is wonderful to experience the scientific method at work, especially when it leads to a scientific discovery!”
Professor Andrews added: ‘‘Professor Valev has led an international team to a real first in the applied photonics. When he invited my participation, it led me back to theory work from my doctoral studies. It has been amazing to see it come to fruition so many years later.”
The research is published in the journal Laser & Photonics Reviews. It was funded by The Royal Society, the Science and Technology Facilities Council (STFC) and the Engineering and Physical Science Research Council (EPSRC). Read full paper. |
 |
| 2D sheets intersect and twist on top of each other, modifying the energy landscape of the materials (Credit: Ventsislav Valev). |
|
Parallel universes cross in Flatland
10 May 2021 University
of Bath
Physicists at the University of Bath observe modified energy landscapes at the intersection of 2D materials.
In 1884, Edwin Abbott wrote the novel Flatland: A Romance in Many Dimensions as a satire of Victorian hierarchy. He imagined a world that existed only in two dimensions, where the beings are 2D geometric figures. The physics of such a world is somewhat akin to that of modern 2D materials, such as graphene and transition metal dichalcogenides, which include tungsten disulfide (WS2), tungsten diselenide (WSe2), molybdenum disulfide (MoS2) and molybdenum diselenide (MoSe2).
Modern 2D materials consist of single-atom layers, where electrons can move in two dimensions but their motion in the third dimension is restricted. Due to this 'squeeze', 2D materials have enhanced optical and electronic properties that show great promise as next-generation, ultrathin devices in the fields of energy, communications, imaging and quantum computing, among others.
Typically, for all these applications, the 2D materials are envisioned in flat-lying arrangements. Unfortunately, however, the strength of these materials is also their greatest weakness – they are extremely thin. This means that when they are illuminated, light can interact with them only over a tiny thickness, which limits their usefulness. To overcome this shortcoming, researchers are starting to look for new ways to fold the 2D materials into complex 3D shapes.
In our 3D universe, 2D materials can be arranged on top of each other. To extend the Flatland metaphor, such an arrangement would quite literally represent parallel worlds inhabited by people who are destined to never meet.
Now, scientists from the Department of Physics at the University of Bath have found a way to arrange 2D sheets of WS2 (previously created in their lab) into a 3D configuration, resulting in an energy landscape that is strongly modified when compared to that of the flat-laying WS2 sheets. This particular 3D arrangement is known as a 'nanomesh': a webbed network of densely-packed, randomly distributed stacks, containing twisted and/or fused WS2 sheets.
Modifications of this kind in Flatland would allow people to step into each other's worlds. "We didn't set out to distress the inhabitants of Flatland," said Professor Ventsislav Valev who led the research, "But because of the many defects that we nanoengineered in the 2D materials, these hypothetical inhabitants would find their world quite strange indeed.
"First, our WS2 sheets have finite dimensions with irregular edges, so their world would have a strangely shaped end. Also, some of the sulphur atoms have been replaced by oxygen, which would feel just wrong to any inhabitant. Most importantly, our sheets intersect and fuse together, and even twist on top of each other, which modifies the energy landscape of the materials. For the Flatlanders, such an effect would look like the laws of the universe had suddenly changed across their entire landscape."
Dr Adelina Ilie, who developed the new material together with her former PhD student and post-doc Zichen Liu, said: "The modified energy landscape is a key point for our study. It is proof that assembling 2D materials into a 3D arrangement does not just result in 'thicker' 2D materials – it produces entirely new materials. Our nanomesh is technologically simple to produce, and it offers tunable material properties to meet the demands of future applications."
Professor Valev added: "The nanomesh has very strong nonlinear optical properties – it efficiently converts one laser colour into another over a broad palette of colours. Our next goal is to use it on Si waveguides for developing quantum optical communications."
PhD student Alexander Murphy, also involved in the research, said: "In order to reveal the modified energy landscape, we devised new characterisation methods and I look forward to applying these to other materials. Who knows what else we could discover?"
The research is published in the journal Laser & Photonics Reviews. It was funded by The Royal Society, the Science and Technology Facilities Council (STFC), the Engineering and Physical Science Research Council (EPSRC) and the UK Quantum Technology Hub Sensors and Timing. |
 |
| The twist of a single nanoparticle, floating freely in a liquid. Credit Ventsislav Valev and Joel Collins. |
|
A plot twist in pharmaceuticals: single nanoparticles could pave the way for medicines on demand
20 July 2020 University
of Bath
For the first time, a single, twisted nanoparticle has been accurately measured and characterised in a lab.
RFor the first time, a single, twisted nanoparticle has been accurately measured and characterised in a lab, taking scientists one vital step closer to a time when medicines will be produced and blended on a microscopic scale.
Physicists at the University of Bath who study materials on the nanoscale – that is, molecules 10,000 smaller than a pinhead – made their groundbreaking observations using a new method for examining the shape of nanoparticles in 3D. This technique, called the hyper-Rayleigh scattering optical activity (HRS OA) technique, was used to examine the structure of gold (among other materials), resulting in an exceptionally clear image of the ‘screw thread’ twist in the metal’s shape.
Understanding the twists within a material (known as its chirality) is vital in industries that produce medicines, perfumes, food additives and pesticides, as the direction in which a molecule twists determines some of its properties. For instance, a molecule that twists clockwise will produce the smell of lemons while the identical molecule twisting anticlockwise (the mirror image of the lemon-smelling molecule) smells of oranges.
“Chirality is one of the most fundamental properties of nature. It exists in sub-atomic particles, in molecules (DNA, proteins), in organs (the heart, the brain), in bio-materials (such as seashells), in storm clouds (tornadoes) and in the shape of galaxies (spirals hurling through space).” said Professor Ventsislav Valev, who led the project.
Until now, physicists have relied on 200-year-old optical methods for determining the chiral properties of molecules and materials, but these methods are weak and require large amounts of molecules or materials to work. Through their use of a technique based on powerful laser pulses, Professor Valev and his team at Bath’s Centre for Photonics and Photonic Materials have produced a far more sensitive probe for chirality, one that can detect a single nanoparticle as it floats freely in a liquid.
This discovery was made by Bath’s Department of Physics in collaboration with the Department of Chemistry. The researchers’ findings are published in Nano Letters.
“This is both a record and a milestone in nanotechnology,” said Professor Valev. “Pursuing this line of research has been one of the most rewarding achievements in my career.”
“The observation by Valev’s group is historic, and scientifically it inspires us in our work to synthesise new chiral 3D nanomaterials,” said study co-author Professor Ki Tae Nam from Material Science and Engineering at the Seoul National University in Republic of Korea.
The potential applications for ultra-sensitive chiral sensing are many. For instance, many pharmaceuticals are chiral. Local pharmacists will be able to harness the technology to mix substances in a completely new way, producing pharmaceuticals from minute droplets of active ingredients rather than from large beakers of chemicals.
“You’ll be able to go to the chemist with a prescription and instead of receiving a medicine that has to be mixed from bottles of chemicals and then stored in the fridge for several days, you’ll walk away with pills that are mini-labs. Upon cracking the pill, a precise number of micro-droplets will flow through microchannels to mix and produce the needed medicine.” said Professor Valev.
“For these mini-labs to produce chiral drugs, you’ll need to know the number of molecules and catalysts within each micro droplet, as well as their chirality.” said PhD student Lukas Ohnoutek, who is the first author on the paper. “This is where our result is really important. We can now aim to produce microdroplets containing a single chiral nanoparticle, to use as catalysts in chemical reactions.”
Professor Valev added: “Looking ahead, we can imagine building up chiral materials and even machines, one nanoparticle at a time, from such microdroplets. To do so would be amazing.” |
 |
| Applications of metal nanostructures. Credit Ventsislav Valev and Alex Murphy. |
|
Dialling up the heat on nanoparticles
21 January 2020 University
of Bath
The ability of metallic nanoparticles to harvest and control light is transforming scientific research, according to physicists from the University of Bath.The ability of metallic nanoparticles to harvest and control light is transforming scientific research, according to physicists from the University of Bath.
Rapid progress in the field of metallic nanotechnology is sparking a science revolution that is likely to impact all areas of society, according to professor of physics Ventsislav Valev and his team at the University of Bath.
Metallic nanotechnology is an area that allows microscopic particles of metals, such as gold and silver, to be manipulated with heat and light. The potential applications are vast, ranging from optimising the way we harvest renewable energy to overhauling our treatment of cancerous tumours.
Writing in Advanced Optical Materials, Prof Valev’s team reviews the current state of nanotechnology research and discusses its likely applications in the near and medium future.
PhD student Lukas Ohnoutek sees nanomedicine – a branch of medicine that uses nanotechnology to improve the diagnosis and treatment of disease – as a particularly buoyant area of research. On-command delivery of drugs has already proven successful in several animal trials, he says. Using this technology, medicines encapsulated in nanomaterials are directed to a specific site in the body before releasing their active ingredients in a highly controlled manner.
“It is crucial to increase the efficiency of drugs and to reduce side effects, and this is something that can be achieved with on-command drug delivery,” said Mr Ohnoutek. “By illuminating metal nanoparticles, it is possible to control the location, time, and amount of drug released in a patient.”
Research fellow Dr Kristina Rusimova says dramatic improvements are expected in the treatment of cancer, thanks both to on-command drug delivery and photothermal cancer therapy (PTT). PTT involves injecting nanoparticles into a patient’s body, where they accumulate in the tumour. When the particles are then subjected to radiation, they heat up and destroy the tumour with very little damage to surrounding tissue. In animal trials, advanced tumours have completely disappeared following photothermal therapy.
“We have looked at animal trials conducted on mice, cats and dogs,” said Dr Rusimova. “In each case, the treatment seemed successful, which is very encouraging for treatment in humans. We know that human trials have been approved and are currently ongoing, so we are cautiously optimistic.”
Other research is focused on finding nanotechnology solutions to the climate crises. There is hope that non-radiative plasmonic decay will provide a new method for improving solar cells and for producing hydrogen fuel directly from water. This process is known as ‘water splitting’. The result will be an efficient and economical low-carbon fuel, particularly suitable for heating homes and other spaces.
Other tantalising applications for metallic nanoparticles technology include advanced biomedical imaging, improved magnetic storage and nanorobotics, where robots are manufactured with components on the nanoscale.
Prof Valev said: “The tiniest metal pieces can now be formed, cut and joined with light. This allows us to integrate humanity’s knowledge of metal working with our understanding of molecular self-assembly and nanoscale biotechnology. This research field offers some truly amazing perspectives for the future.”
These prospects are all built around the ability of metallic nanoparticles to harvest and control light at the subwavelength scale. |
 |
| Shedding light on a nanomaterial that both twisted and not at the same time. Credit Ventsislav Valev and Alex Murphy |
|
Scientists illuminate secrets of a nanomaterial that is both twisted and untwisted at the same time
13 September 2019 University
of Bath
The material developed at University of Bath allows for incredibly sensitive detection of the direction molecules twist.
A new nanomaterial developed by scientists at the University of Bath could solve a conundrum faced by scientists probing some of the most promising types of future pharmaceuticals.
Scientists who study the nanoscale - with molecules and materials 10,000 smaller than a pinhead - need to be able to test the way that some molecules twist, known as their chirality, because mirror image molecules with the same structure can have very different properties. For instance one kind of molecule smells of lemons when it twists in one direction, and oranges when twisted the other way.
Detecting these twists is especially important in some high-value industries such as pharmaceuticals, perfumes, food additives and pesticides.
Recently, a new class of nanoscale materials have been developed to help distinguish the chirality of molecules. These so-called ‘nanomaterials’ usually consist of tiny twisted metal wires, that are chiral themselves.
However, it has become very hard to distinguish the twist of the nanomaterials from the twist of the molecules they are supposed to help study.
To solve this problem the team from the University of Bath’s Department of Physics created a nanomaterial that is both twisted and it is not. This nanomaterial has equal number of opposite twists - meaning they cancel each other out. Usually, upon interacting with light, such material appears without any twist; how then could it be optimised to interact with molecules? Using a mathematical analysis of the material’s symmetry properties, the team discovered a few special cases, which can bring the ‘hidden’ twist to light and allow very sensitive detection of chirality in molecules.
Lead author Professor Ventsislav Valev, from the University of Bath Department of Physics, said: “This work removes an important roadblock for the entire research field and paves the way to ultra-sensitive detection of chirality in molecules, using nanomaterials.”
PhD student Alex Murphy, who worked on the study, said: “Molecular chirality is an amazing property to study. You can smell chirality, since the same but oppositely twisted molecules smell of lemons and oranges. You can taste chirality, since one twist of Aspartame is sweet and the other is tasteless. You can feel chirality, since one twist of menthol gives a cool sensation to the skin while the other does not. You touch chirality expressed in the twist of seashells. And it is great to see chirality expressed in its interactions with the colours of laser light.”
The study is published in the journal Nanoscale Horizons. The research was funded by the Royal Society, the Engineering and Physical Sciences Research Council, and the Science and Technology Facilities Council. |
 |
| Green laser light is absorbed by nanoparticles and converted into heat. Credit Ventsislav Valev & Kristina Rusimova |
|
Quantum computing boost from vapour stabilising technique
30 May 2019 University
of Bath
The patented technique allows electrons to be used for quantum computing, high-precision measurements and other applications.
A technique to stabilise alkali metal vapour density using gold nanoparticles, so electrons can be accessed for applications including quantum computing, atom cooling and precision measurements, has been patented by scientists at the University of Bath.
Alkali metal vapours, including lithium, sodium, potassium, rubidium and caesium, allow scientists to access individual electrons, due to the presence of a single electron in the outer ‘shell’ of alkali metals.
This has great potential for a range of applications, including logic operations, storage and sensing in quantum computing, as well as in ultra-precise time measurements with atomic clocks, or in medical diagnostics including cardiograms and encephalograms.
However, a serious technical obstacle has been reliably controlling the pressure of the vapour within an enclosed space, for instance the tube of an optical fibre. The vapour needs to be prevented from sticking to the sides in order to retain its quantum properties, but existing methods to do this, including directly heating vapour containers are slow, costly, and impractical at scale.
Scientists from the University of Bath, working with a colleague at the Bulgarian Academy of Sciences, have devised an ingenious method of controlling the vapour by coating the interior of containers with nanoscopic gold particles 300,000 times smaller than a pinhead.
When illuminated with green laser light the nanoparticles rapidly absorb and convert the light into heat, warming the vapour and causing it to disperse into the container more than 1,000 times quicker than with other methods. The process is highly reproducible and, in addition, the new nanoparticle coating was found to preserve the quantum states of alkali metal atoms that bounce from it.
The study is published in Nature Communications.
Professor Ventsislav Valev, from the University of Bath’s Department of Physics led the research. He said: “We are very excited by this discovery because it has so many applications in current and future technologies! It would be useful in atomic cooling, in atomic clocks, in magnetometry and in ultra-high-resolution spectroscopy.
“Our coating allows fast and reproducible external control of the vapour density and related optical depth, crucial for quantum optics in these confined geometries.”
Assoc. Prof Dimitar Slavov, from the Institute of Electronics in the Bulgarian Academy of Sciences, added “In this proof of principle, it was demonstrated that illuminating our coating significantly outperforms conventional methods and is compatible with standard polymer coatings used to preserve quantum states of single atoms and coherent ensembles.”
Dr Kristina Rusimova, a prize fellow in the Department of Physics, added: “Further improvements of our coating are possible by tuning particle size, material composition and polymer environment. The coating can find applications in various containers, including optical cells, magneto-optical traps, micro cells, capillaries and hollow-core optical fibres.”
The research was funded by the Engineering and Physical Sciences Research Council (EPSRC) UK Quantum Technology Hub “Networked Quantum Information Technologies” and the Royal Society. |
 |
| Clean air over Shanghai. Credit Ventsislav Valev |
|
Electrode's 'hot edges' convert CO2 gas into fuels and chemicals
15 May 2019 University
of Bath
The bowl-shaped design can efficiently convert CO2 from gas into carbon based fuels and chemicals, helping combat the threat of atmospheric carbon dioxide.
A team of scientists has created a bowl-shaped electrode with ‘hot edges’ which can efficiently convert CO2 from gas into carbon based fuels and chemicals, helping combat the climate change threat posed by atmospheric carbon dioxide.
The research team, from the University of Bath, Fudan University, Shanghai, and the Shanghai Institute of Pollution Control and Ecological Security, hopes the catalyst design will eventually allow the use of renewable electricity to convert CO2 into fuels without creating additional atmospheric carbon - essentially acting like an electrochemical ‘leaf’ to convert carbon dioxide into sugars.
Using this reaction, known as the reduction of carbon dioxide, has exciting potential but two major obstacles are poor conversion efficiency of the reaction and a lack of detailed knowledge about the exact reaction pathway.
This new electrode addresses these challenges with higher conversion efficiency and sensitive detection of molecules created along the reaction’s progress – thanks to its innovative shape and construction. The bowl shaped electrode works six times faster than standard planar - or flat - designs.
The bowl-like shape of the design, technically known as an “inverse opal structure” concentrates electric fields on its hot edges - the rim of the bowl - which then concentrates positively charged potassium ions on the active sites of the reaction, reducing its energy requirements.
The Copper-Indium alloy electrode can also be useful to sensitively study the reaction process via measuring the Raman signal, which is higher compared to a typical electrode.
The study is published in the Royal Society of Chemistry’s Journal of Materials Chemistry A.
Professor Ventsislav Valev, from the University of Bath’s Department of Physics, said: “There is no more pressing human need than breathing. Yet for hundreds of million people this most basic activity is a source of anxiety over lowering life expectancy, rising child mortality and climate change. There is evidence that CO₂ increases surface ozone, carcinogens, and particulate matter, thereby increasing death, asthma, hospitalization, and cancer rates. It is therefore crucial to keep researching new ways for lowing the CO₂ levels in the atmosphere.”
The team wants to continue research to develop the most efficient catalyst to perform carbon reduction.
Professor Liwu Zhang, from Fudan University, said: “CO2 is causing climate change, making our planet warmer. By using clean electricity, we can convert CO2 into chemical fuels, which can be used again. This builds a cycle of CO2, with no increment of CO2 concentration and will help save our world.
“However, to improve the efficiency of transforming CO2 into chemical fuels, it is extremely important to know the reaction pathway, and find the most suitable catalyst.
“Just as plants transform CO₂ into sugar we are finding suitable electrochemical ‘leaf’ for CO₂ conversion.”
The study: "Hot edges" in an inverse opal structure enable efficient CO2 electrochemical reduction and sensitive in situ Raman characterization” is published in Journal of Materials Chemistry A.
The study was funded by the Ministry of Science and Technology of the People's Republic of China, and National Natural Science Foundation of China, the Engineering and Physical Sciences Research Council(EPSRC) Centre for Doctoral Training in Condensed Matter Physics (CDT-CMP), and the Royal Society.
Read the research paper. |
 |
| The nanomesh's properties mean it can change the colour of laser light. Credit Ventsislav Valev and Alex Murphy |
|
New tunable nanomaterials possible via flexible process invented by University of Bath physicists
11 April 2019 University
of Bath
Physicists have developed a flexible way to synthesise a wide range of novel nanomaterials with potential applications in areas including optics and sensors.
Physicists at the University of Bath have developed a flexible process allowing the synthesis in a single flow of a wide range of novel nanomaterials with various morphologies, with potential applications in areas including optics and sensors.
The nanomaterials are formed from Tungsten Disulphide - a Transition Metal Dichalcogenide (TMD) - and can be grown on insulating planar substrates without requiring a catalyst. TMDs are layered materials, and in their two-dimensional form can be considered the inorganic analogues of graphene.
The various Tungsten Disulphide morphologies synthesized - two-dimensional sheets growing parallel to the substrate, nanotubes, or a nanomesh resembling a ‘field of blades’ growing outwards from the substrate - are possible due to Dr. Zichen Liu’s PhD research at Bath to split the growth process into two distinct stages. Through this decoupling, the growth process could be routed differently than in more conventional approaches, and be guided to produce all these material morphologies.
So far, the ‘field of blades’ morphology has shown powerful optical properties, including strong non-linear effects such as Second Harmonic Generation, that is, doubling the frequency and halving the wavelength of laser light, changing its colour as it does so. The strength of these effects opens up a range of optical applications for the material.
The research is published in ACS Nano.
Dr Adelina Ilie, from the University of Bath’s Department of Physics, who led the research, said: “The simplicity of this process is important from the standpoint that it allows us to obtain practically all phases of this Transition Metal Dichalcogenide, from in-plane to out-of-plane, as well as from two-dimensional sheets to one-dimensional nanotubes and everything between. Usually different processes are used to create two-dimensional or one-dimensional morphologies. Our process, instead, leads to tunable materials with tunable properties.
“The ‘field of blades’ morphology is entirely new, and due to its very large effective surface area, might be of interest not only for the non-linear optical properties we showed so far, but also for application in various sensing technologies. We are exploring all these avenues now.”
Professor Ventsislav Valev, who tested the nanomesh for optical properties added: “We haven’t actually been able to test the upper limits of the optical effects yet because the signal is too strong for the equipment we used to probe it. We are talking about a material that is one or two atoms in thickness; it is quite extraordinary. Its arrangement into a ‘field of blades’ clearly increases the signal.”
The team plans to continue to explore the properties of the materials.
The research was supported by the University of Bath, the Engineering and Physical Sciences Research Council, and the Royal Society.
Read the reseach paper. |
 |
| L-R: Kristina Rusimova, Joel Collins, Ventsislav Valev and David Hooper |
|
New physical effect demonstrated by University of Bath scientists after a 40 year search
6 February 2019 University
of Bath
The discovery could lead to advancements in diverse fields including chemical manufacturing, pharmaceuticals and miniaturisation
A new physical effect has been demonstrated at the University of Bath after 40 years of pursuit by physicists around the world, which could lead to advancements in chemical manufacturing efficiency, miniaturisation and quality control in personalised pharmaceuticals.
For the first time ever the research team in the Department of Physics was able to use a physical effect - specifically the colour-changing of light scattered from chiral molecules - to measure the chirality present, confirming predictions of theoretical work from the 1970s. The technique is 100,000 times more sensitive than standard methods used today.
Chirality describes the orientation of molecules, which can exist in left or right ‘handed’ forms depending on how they twist in three dimensions. Many molecules essential to life, including DNA, amino acids and proteins, exhibit chirality and the handedness can totally change their function or properties. Therefore knowing the chirality of a substance is often critically important.
For decades scientists had sought to prove that you could accurately determine the chirality of molecules by measuring a colour-changing (nonlinear) effect upon illumination with twisted (circularly polarised) light. In theory, twisted light could change colour and then scatter differently from differently handed molecules - but this had never been demonstrated experimentally.
Dr Ventsislav Valev, who leads the research group in the Department of Physics at the University of Bath, said: “We’ve demonstrated a new physical effect - you don’t get to say that every day. This is exactly why I got into science.
“We began thinking about the problem 13 years ago, together with Prof Thierry Verbiest, at KU Leuven, Belgium. Because the effect was so elusive, I knew half of the solution would be to develop a very sensitive experimental setup. This is what I did for many years. The other half was finding the right samples and I was really excited to discover the nanoscopic silver springs (nano-helices) fabricated by Prof Peer Fischer’s group, at the Max Planck Institute for Intelligent Systems, in Stuttgart, Germany.”
PhD student Joel Collins had an incredible moment when running a series of tests on these springs.
He said: “To be honest my attitude was almost ‘OK let’s get this out the way to make sure it doesn’t work and we can move onto something else’. Then, together with my colleague Dr Kristina Rusimova, we noticed that there did actually seem to be an effect, and I thought ‘Hmmmm, that’s interesting.’
“We kept repeating the experiment to make sure it was actually a real effect and we saw that not only it’s there but it’s huge – we were only using really low concentrations of our nano-helices.
“For my part, I didn’t really recognise how important it is, and was expecting someone to come along and rip it to shreds, to say - ‘you haven’t thought of that’ or ‘you’ve missed this’. But over time it has dawned on me - that this is actually a fantastic result.”
The experimental geometry is in fact quite simple; the nano-springs are dispersed in water within a glass container where they spread randomly. Then a laser is aimed at them. The twist (circular polarisation) of the laser is switched periodically and light scattered from the container at 90° is analysed to determine the chirality of the springs present. The research is published in Physical Review X.
Dr Valev added: “It’s taken 40 years, people have been looking for this without success, and not for lack of trying. It’s amazing. The theory was quite controversial, people thought that maybe the effect was impossible to observe, maybe something else was there, blocking it.
“For 200 years, scientists have been using the same method to measure chirality. It’s not very sensitive, but it’s robust and simple, however precise measurements of chirality have become a major hurdle for human-made chiral nanotechnology because of false positives.
“Now we have a method 100,000 times more sensitive, free from false positives. There’s a new kind of manufacturing process currently emerging. It is called “lab-on-a-chip” and our effect fits very well with it.
“A more sensitive test means you can use lower quantities in quality control and reduce waste, there are applications in chemical and pharmaceutical manufacturing, as well as in microfluidics, in miniaturisation and for developing personal pharmaceutical technologies.”
Advanced laser sources, sensitive detection equipment and state-of-the-art nanofabrication techniques have all come together to enable the experimental observation of the new effect.
Professor David Andrews, from the University of East Anglia, theorised the effect 40 years ago. He said: “Dr Valev’s pioneering work is a clever and highly significant achievement, for he has realised a kind of application that could never have been imagined when the theory was first laid, forty years ago.
“His results serve as an encouragement to all pure theorists!”
Next, the researchers will be using their findings to characterise chiral molecules and to develop its technological applications. The paper “First observation of optical activity in hyper-Rayleigh scattering” is published in Physical Review X. DOI: 10.1103/PhysRevX.9.011024.
The research was funded by The Royal Society, the Science and Technology Facilities Council (STFC) and the Engineering and Physical Science Research Council (EPSRC).
Read the research paper. |
 |
| In the sensor, gold nanodisks are arranged in squares, shown on bottom-left. The arrangement causes the sensor to emit UV light (in blue). (V.K Valev and D.C Hooper) |
|
Ultra-sensitive sensor with gold nanoparticle array
9 January 2019 University
of Bath
Physicists from Bath have developed a new type of sensor platform using a gold nanoparticle array, that's 100 times more sensitive than current similar sensors
The sensor is made up of a series of gold disk-shaped nanoparticles on a glass slide. The team at Bath discovered that when they shone an infra-red laser at a precise arrangement of the particles, they started to emit unusual amounts of ultra violet (UV) light.
This mechanism for generating UV light is affected by molecules binding to the surface of the nanoparticles, providing a means of sensing a very small amount of material.
The researchers, from the University of Bath’s Department of Physics, hope that in the future they can use the technology to develop new ultra-sensitive sensors for air pollution or for medical diagnostics.
Dr Ventsislav Valev, Royal Society Research Fellow and Reader in Physics at the University of Bath, led the work with Research Associate David Hooper.
He explained: “This new mechanism has great potential for detecting small molecules. It is 100 times more sensitive than current methods.
“The gold nanoparticle disks are arranged on a glass slide in a very precise array – changing the thickness and separation of the disks completely changes the detected signal.
“When molecules bind to the surface of a gold nanoparticle, they affect the electrons at the gold surface, causing them to change the amount of UV light they emit.
“The amount of UV light emitted would depend on the type of molecules that bind to the surface.
“This technique could enable ultra-sensitive detection of molecules in tiny volumes. It could in the future be used for detecting very low concentrations of biological markers for the early diagnostic screening for diseases, such as cancer.”
The study has demonstrated the proof of principle for this new sensing mechanism. The team would next like to test the sensing of various types of chemicals and expects the technique to be available to other scientists to use within five years.
The nanoparticles were fabricated by researchers at Northwestern University, Illinois (USA).
David C. Hooper, Christian Kuppe, Danqing Wang, Weijia Wang, Jun Guan, Teri W. Odom, and Ventsislav K. Valev (2018) “Second Harmonic Spectroscopy of Surface Lattice Resonances” is published in Nano Letters DOI: 10.1021/acs.nanolett.8b03574 |
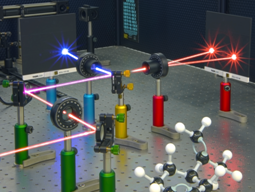 |
| A laser beam changed from red to blue by interacting with chiral molecules. Credit Ventsislav Valev and Joel Collins. |
|
Twisted meta-molecules as they really are
26 June 2018 University
of Bath
Physicists at the University of Bath have devised a new method to truly test the chirality of a material, eliminating the risk of false positives
Physicists at the University of Bath have devised a new and highly sensitive method to truly test the chirality of a material, eliminating the risk of false positives from competing effects.
Chiral molecules exist in different forms, even when they are made of the same atoms, those atoms can be arranged differently – twisting one way or another. This crucial difference can affect the properties of the molecules and has applications in fields such as telecommunications, nanorobotics, pharmaceuticals and industrial chemicals.
However, because of the nanoscopic nature of many of these molecules and materials it can be difficult for scientists to be certain they are working with chiral molecules of a particular twist (known as ‘handedness’). Some tests which are used can produce false positives, meaning scientists could be left working with the wrong samples.
The University of Bath team, working with colleagues at the Max Planck Institute for Intelligent Systems in Germany, demonstrated a method to separate the chirality of a substance from sources of false positives. In order to achieve this they used the way light interacts with artificial molecules (known as ‘meta-molecules’) that were made of tiny gold helices.
The team directed a powerful laser beam at samples of chiral meta-molecules, which caused it to change colour: from red to blue. Because the sample chirality imprints a twist on light, the blue light was also twisted: 45° left or right, depending on the sample handedness. Previously, false positives (such as anisotropy) have been known to affect chiral optical measurements.
Joel Collins, who conducted the experiments said: “Some other techniques can produce a similar effect but without this being due to the chirality which can be misleading. By being able to rotate the sample and retain the optical effects we have a true test of chirality, and you can be sure what you’re seeing is due to chirality and not some other property.”
Dr Ventsislav Valev who led the research said: “Meta-molecules are scientifically very exciting. Our demonstration of this effect, free from anisotropy contributions, is a first in the research field. Plus, we were astounded by how large it was. A 45° twist usually requires travelling through 5 cm of concentrated sugar syrup. Our material is half a million times thinner.”
The research is published in the journal ACS Nano.
The Research was funded by The Royal Society, Science and Technologies Facilities Council (STFC) and the Engineering and Physical Sciences Research Council (EPSRC). |
 |
| An impression of a chiral molecule moving through various configurations as it transitions from one handedness to the other. Credit Ventsislav Valev & Joel Collins |
|
Morphing twisted nanoscale objects opens up a new way to tailor applications in future technologies
10 April 2018 University
of Bath
Scientists have created a way to model interactions between light and twisted molecules, as they transition from left to right-handed versions, or vice versa.
For the first time scientists have created a way to model the interaction between light and twisted molecules, as these molecules transition from left- to right-handed versions, or vice versa. The transitional forms offer a deeper insight into material symmetries and their unexpected behaviour could lead to improved design of telecoms components.
Many molecules, including important pharmaceuticals and valuable chemicals, exist in two “chiral” forms – they have the same chemical structure arranged in mirror images, termed left-handed and right-handed forms. This can alter their properties and is therefore important to fully understand how the compound interacts with other molecules, or light.
Typically, it has only been possible to study either the left- or right-handed chiral form but nothing in between, however ideally scientists would like to gradually morph a shape from one handedness to the other and observe how the effects of this change translate into physical properties.
Now a research team from the Department of Physics at the University of Bath, working with colleagues at University College London, Belgium and China, has created a way to do exactly that.
Their unique method involves manufacturing metallic nano-scale “artificial molecules” representative of 35 intermediate stages along the way of a geometric transformation, from one handedness to the other. At this nano-scale, the shape of the artificial molecule affects its optical properties, so by using twisted laser light the team studied the properties of the various stages, as the artificial molecules morphed from left to right handedness.
PhD student Joel Collins said: “We were able to follow the properties of a chiral artificial molecule, as it was morphed from left- to right-handed form, through two different routes. No-one has done this before. Surprisingly, we found that each route leads to a different behavior.
"We measured the difference in absorption of left and right circularly polarized light, known as circular-dichroism (CD). Along one route, the artificial molecules behaves as might be expected, with progressively decreasing CD, and eventually a reversal of the CD, for the mirrored structure. However, along the second route, the CD reversed several times, even before the structure changed handedness.”
The research is published in the journal Advanced Optical Materials.
Dr Ventsislav Valev who led the research said: “This is actually a very elegant idea but it has only become a possibility thanks to the recent advances in nanofabrication.
“In chemistry, you can’t tune the twist of a chiral molecule, so every scientist who studies such molecules needs to tune the wavelength of light. We have demonstrated a new, complementary physical effect, where we fix the wavelength and tune the twist of the chiral artificial molecule. In many cases, our approach is more practical; for instance, when we’re designing telecoms components, where the optical wavelength is pre-determined.”
The research was funded by the Royal Society and the Engineering and Physical Sciences Research Council. |
 |
| Twisting laser light can help study nanoscopic molecules. Credit Ventsislav Valev. |
|
Twisting laser light to probe the nanoscale
05 April 2018 University
of Bath
A new method to sensitively measure the structure of molecules has been demonstrated by twisting laser light and aiming it at miniscule gold gratings.
A new method to sensitively measure the structure of molecules has been demonstrated by twisting laser light and aiming it at miniscule gold gratings to separate out wavelengths.
The technique could potentially be used to probe the structure and purity of molecules in pharmaceuticals, agrochemicals, foods and other important products more easily and cheaply than existing methods.
Developed by physicists at the University of Bath, working with colleagues at the University of Cambridge and University College London, the technique relies on the curious fact that many biological and pharmaceutical molecules can be either ‘left-handed’ or ‘right-handed’. Although such molecules are built from exactly the same elements they can be arranged in mirror images of each other, and this configuration sometimes changes their properties drastically.
Notoriously the morning sickness drug Thalidomide caused birth defects and deaths in babies before it was pulled from the market in the 1960s. Investigation showed that the drug existed in two mirror images – the right-handed form was effective as a morning sickness drug, but the left-handed form was harmful to foetuses. This is one example of why testing what ‘handedness’, or chirality, a molecule has is essential for many valuable products.
The research team from the Centre for Photonics and Photonic Materials, and the Centre for Nanoscience and Nanotechnology at the University of Bath, used a special white-light laser built in-house and directed it through several optical components to put a twist on the beam. The twisted laser beam then hits a nano-scopic U-shaped gold grating which serves as a template for the light, further twisting the beam in either a right or left-handed direction. This deflects the beam in many directions and further splits it into its constituent wavelengths across the colour spectrum.
By carefully measuring the deflected light scientists can detect tiny differences in intensity across the spectrum which inform them about the chirality of the grating the laser beam interacts with.
The study, published in the journal Advanced Optical Materials, demonstrates the technique as a proof of principle.
Christian Kuppe, the PhD student who conducted the experiments, said: “At the moment chiral sensing requires high molecular concentrations because you’re looking for tiny differences in how the light interacts with the target molecule.
“By using our gold gratings we aim to use a much smaller amount of molecules to conduct a very sensitive test of their handedness. The next step will be to continue to test the technique with a range of well-known chiral molecules.
“We hope that this will become a valuable way to perform really important tests on all sorts of products including pharmaceuticals and other high-value chemicals.”
Dr Ventsislav Valev, who oversaw the work, said: “There’s a great deal of scientific excitement about miniaturisation and working on nano-sized dimensions at the very small scale. However, in the rush to go as small as possible, some opportunities have been overlooked. Working with chiral nano-gratings is a great example of that.”
The researchers were funded by grants from the Royal Society, European Research Council, China Scholarship Council and Engineering and Physical Sciences Research Council (EPSRC). |
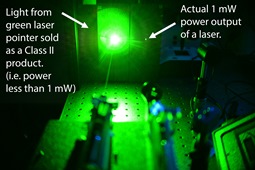 |
| Some lasers are much more powerful than advertised. Credit Ventsislav Valev. |
|
Public unwittingly buying dangerous laser pointers warn scientists
09 October 2017 University
of Bath
Dangerous laser pointers are being sold to the public which unwittingly believes them to be safe, scientists from the University of Bath have warned.
Misleading and mistaken labelling, drastic variations in laser strength under different conditions, and emissions of invisible infrared light all pose risks they say.
The researchers have responded to a Government consultation on laser pointers calling for better labelling and new easy-to-understand warning signs for all laser pointers.
Physicist Dr Ventsislav Valev, who has experienced a laser pointer attack, and who led tests at the University of Bath on a range of laser pointers, said: “Our main concern is laser pointers which seem safe to the user but are in fact very powerful. These lasers can lull people into a false sense of security and lead to injury.
“Some lasers that are labelled as safe turn out to be highly dangerous because they can emit highly powerful invisible laser light. Moreover, the visible laser power can vary a lot, depending on temperature.
“Other labels are mistaken, and the maximum power output of the laser is often higher than the safety class on the label, again putting the user and others in danger.”
Dr Valev and PhD student Christian Kuppe in the University of Bath Department of Physics, collaborated with Dr Dimitar Slavov from the Bulgarian Academy of Sciences to develop scientific equipment for carefully testing the laser diodes in the heart of every pointer.
“Our results show that the so-called ‘frequency doubled’ laser pointers, usually green, blue and violet pointers, can be particularly dangerous even if they seem safe to the user,” he said.
“For example, some laser pointers can output widely different laser power depending on the temperature. They can appear perfectly safe at room temperature only to become much more dangerous outside and vice-versa. Moreover, as pointers are being used they heat up, so a pointer that initially seems safe can subsequently become highly powerful and dangerous.
“Other lasers can produce safe levels of coloured light, but at the same time emit high power invisible infrared light. A person looking at the visible green light would estimate the laser to be safe and the much greater power and danger would go unnoticed until injury occurs.”
Laser pointers have been controversial, in particular because they have been shined into the eyes of plane and helicopter pilots and train drivers, with an average of 1,500 reported attacks per year in the UK. They can cause permanent or temporary eye damage, and it is a criminal offence to do so.
Dr Valev added: “I know from personal experience what it is like to be the victim of a laser pointer attack. The circumstances were particularly aggravating as I had my baby daughter in my arms at the time and so she was exposed too. Fortunately she was just falling asleep so she had her eyes closed.
“The pointer was powerful, but poor quality so the laser spot was about a meter in size when it reached me and my daughter. Because of this huge spread, the amount of laser light entering my eyes was much reduced from the original laser pointer output. So, I got only momentarily dazed, but suddenly everything became red. I was thinking that perhaps I was experiencing a medical condition, but my wife saw someone shining a pointer at me from outside our home.
“Then I got very angry that someone would direct a high power laser pointer at a person holding a baby. These are not toys and can be very dangerous, especially to babies’ eyes. We need to make sure laser pointers being sold are properly labelled and safe to use.” |
 |
| Gold spring shaped coils help reveal information about chiral molecules. Credit Ventsislav Valev. |
|
Nanoscopic golden springs could unravel twisted molecules
03 March 2017 University
of Bath
University of Bath scientists have used gold spring-shaped coils 5,000 times thinner than human hairs and powerful lasers to enable the detection of twisted molecules, and the applications could improve pharmaceutical design, telecommunications and nanorobotics. Molecules, including many pharmaceuticals, twist in certain ways and can exist in left or right ‘handed’ forms depending on how they twist. This twisting, called chirality, is crucial to understand because it changes the way a molecule behaves, for example within our bodies.
Scientists can study chiral molecules using particular laser light, which itself twists as it travels. Such studies get especially difficult for small amounts of molecules. This is where the minuscule gold springs can be helpful. Their shape twists the light and could better fit it to the molecules, making it easier to detect minute amounts.
Using some of the smallest springs ever created, the researchers from the University of Bath Department of Physics, working with colleagues from the Max Planck Institute for Intelligent Systems, examined how effective the gold springs could be at enhancing interactions between light and chiral molecules. They based their study on a colour-conversion method for light, known as Second Harmonic Generation (SHG), whereby the better the performance of the spring, the more red laser light converts into blue laser light.
They found that the springs were indeed very promising but that how well they performed depended on the direction they were facing.
Physics PhD student David Hooper who is the first author of the study, said: “It is like using a kaleidoscope to look at a picture; the picture becomes distorted when you rotate the kaleidoscope. We need to minimise the distortion.”
In order to reduce the distortions, the team is now working on ways to optimise the springs, which are known as chiral nanostructures.
“Closely observing the chirality of molecules has lots of potential applications, for example it could help improve the design and purity of pharmaceuticals and fine chemicals, help develop motion controls for nanorobotics and miniaturise components in telecommunications,” said Dr Ventsislav Valev who led the study and the University of Bath research team.
The research is published in the journal Advanced Materials.
The research is funded by the Royal Society as part of a the University Research Fellowships scheme, as well as by the UK Engineering and Physical Sciences Research Council (EPSRC) through the Centre for Doctoral Training in Condensed Matter Physics.
Full bibliographic information
D. C. Hooper, A. G. Mark, C. Kuppe, J. T. Collins, P. Fischer, V. K. Valev, Strong Rotational Anisotropies Affect Nonlinear Chiral Metamaterials Adv. Mater. 29, 1605110 (2017). |
 |
An efficient route to manufacturing nanomaterials with light
through plasmon-induced laser-threading of gold nanoparticle
strings - See more at: http://www.cam.ac.uk/research/news/building-invisible-materials-with-light#sthash.pxt4IcNo.dpuf
An efficient route to manufacturing nanomaterials with light
through plasmon-induced laser-threading of gold nanoparticle
strings - See more at: http://www.cam.ac.uk/research/news/building-invisible-materials-with-light#sthash.pxt4IcNo.dpuf
Expanding polymer-coated gold
nanoparticles, Yi Ju University of Cambridge. |
|
Little ANTs: researchers build the world's tiniest
engine.
02 May 2016 University
of Bath
Researchers have developed the world's
tiniest engine - just a few billionths of a metre in size - which uses light
to power itself. The nanoscale engine, developed by scientists at the
University of Cambridge and University of Bath, could form the basis of
future nano-machines that can navigate in water, sense the environment
around them, or even enter living cells to fight disease.
The prototype device is made of tiny charged
particles of gold, bound together with temperature-responsive polymers in
the form of a gel. When the 'nano-engine' is heated to a certain temperature
with a laser, it stores large amounts of elastic energy in a fraction of a
second, as the polymer coatings expel all the water from the gel and
collapse. This has the effect of forcing the gold nanoparticles to bind
together into tight clusters. But when the device is cooled, the polymers
take on water and expand, and the gold nanoparticles are strongly and
quickly pushed apart, like a spring. The results are reported in the journal
PNAS.
"We know that light can heat up water to
power steam engines," said study co-author Dr Ventsislav Valev, from the
University of Bath's Department of Physics. "But now we can use light to
build a piston for engines at the nanoscale."
"It's like an explosion," said Dr Tao Ding
from Cambridge's Cavendish Laboratory, and the paper's first author. "We
have hundreds of gold balls flying apart in a millionth of a second when
water molecules inflate the polymers around them."
Nano-machines have long been a dream of
scientists and public alike, but have remained in the realm of science
fiction since ways to manufacture them hadn't been developed. The new method
developed by the researchers from Cambridge and Bath is incredibly simple,
yet can be extremely fast and exert large forces.
The forces exerted by these tiny devices are
several orders of magnitude larger than those for any other previously
produced device, with a force per unit weight nearly a hundred times better
than any motor or muscle. According to the researchers, the devices are also
bio-compatible, cost-effective to manufacture, fast to respond, and energy
efficient.
Professor Jeremy Baumberg from the Cavendish
Laboratory, who led the research, has named the devices 'ANTs', or actuating
nano-transducers. "Like real ants, they produce large forces for their
weight. The challenge we now face is how to control that force for nano-machinery
applications."
The research suggests how to turn Van de
Waals energy - the attraction between atoms and molecules - into elastic
energy of polymers and release it very quickly. "The whole process is like a
nano-spring," said Baumberg. "The smart part here is we make use of Van de
Waals attraction of heavy metal particles to set the springs (polymers) and
water molecules to release them, which is very reversible and reproducible."
The team is currently working with Cambridge
Enterprise, the University's commercialisation arm, and several other
companies with the aim of commercialising this technology for microfluidics
bio-applications.
The research is funded as part of a UK
Engineering and Physical Sciences Research Council (EPSRC) investment in the
Cambridge NanoPhotonics Centre, as well as the European Research Council (ERC).
Dr Valev's research is supported by The Royal Society through the University
Research Fellowships.
91 per cent of physics research from the
University of Bath was judged to be world-leading or internationally
excellent by the in the recent independently-assessed Research Excellence
Framework 2014.
Full bibliographic information
T. Ding, V. K. Valev, A. R. Salmon, C. J.
Forman, S. K. Smoukov, O. A. Scherman, D. Frenkel, J. J. Baumberg,
Light-induced actuating nanotransducers: ANTs. PNAS 113, 5503-5507 (2016). |
 |
An efficient route to manufacturing nanomaterials with light
through plasmon-induced laser-threading of gold nanoparticle
strings - See more at: http://www.cam.ac.uk/research/news/building-invisible-materials-with-light#sthash.pxt4IcNo.dpuf
An efficient route to manufacturing nanomaterials with light
through plasmon-induced laser-threading of gold nanoparticle
strings - See more at: http://www.cam.ac.uk/research/news/building-invisible-materials-with-light#sthash.pxt4IcNo.dpuf
An efficient route to manufacturing
nanomaterials with light through plasmon-induced laser-threading
of gold nanoparticle strings. |
|
A new technique which uses light like a needle to
thread long chains of particles could help bring sci-fi concepts such as
cloaking devices one step closer to reality.
28 June 2014 University
of Cambridge
A new method of building materials using
light, developed by researchers at the University of Cambridge, could one
day enable technologies that are often considered the realm of science
fiction, such as invisibility cloaks and cloaking devices.
Although cloaked starships won't be a
reality for quite some time, the technique which researchers have developed
for constructing materials with building blocks a few billionths of a metre
across can be used to control the way that light flies through them, and
works on large chunks all at once. Details are published today (28 July) in
the journal Nature Communications.
The key to any sort of "invisibility" effect
lies in the way light interacts with a material. When light hits a surface,
it is either absorbed or reflected, which is what enables us to see objects.
However, by engineering materials at the nanoscale, it is possible to
produce "metamaterials": materials which can control the way in which light
interacts with them. Light reflected by a metamaterial is refracted in the "wrong" way, potentially rendering objects invisible, or making them appear
as something else.
Metamaterials have a wide range of potential
applications, including sensing and improving military stealth technology.
However, before cloaking devices can become reality on a larger scale,
researchers must determine how to make the right materials at the nanoscale,
and using light is now shown to be an enormous help in such nano-construction.
The technique developed by the Cambridge
team involves using unfocused laser light as billions of needles, stitching
gold nanoparticles together into long strings, directly in water for the
first time. These strings can then be stacked into layers one on top of the
other, similar to Lego bricks. The method makes it possible to produce
materials in much higher quantities than can be made through current
techniques.
In order to make the strings, the
researchers first used barrel-shaped molecules called cucurbiturils (CBs).
The CBs act like miniature spacers, enabling a very high degree of control
over the spacing between the nanoparticles, locking them in place.
In order to connect them electrically, the
researchers needed to build a bridge between the nanoparticles. Conventional
welding techniques would not be effective, as they cause the particles to
melt. "It's about finding a way to control that bridge between the nanoparticles," said Dr Ventsislav Valev of the University's Cavendish
Laboratory, one of the authors of the paper. "Joining a few nanoparticles
together is fine, but scaling that up is challenging."
The key to controlling the bridges lies in
the cucurbiturils: the precise spacing between the nanoparticles allows much
more control over the process. When the laser is focused on the strings of
particles in their CB scaffolds, it produces plasmons: ripples of electrons
at the surfaces of conducting metals. These skipping electrons concentrate
the light energy on atoms at the surface and join them to form bridges
between the nanoparticles. Using ultrafast lasers results in billions of
these bridges forming in rapid succession, threading the nanoparticles into
long strings, which can be monitored in real time.
"We have
controlled the dimensions in a way that hasn't been possible before," said
Dr Valev, who worked with researchers from the Department of Chemistry, the
Department of Materials Science & Metallurgy, and the Donostia International
Physics Center in Spain on the project. "This level of control opens up a
wide range of potential practical applications."
Full bibliographic information
L. O. Herrmann, V. K. Valev, C. Tserkezis, J.
S. Barnard, S. Kasera, O. A. Scherman, J. Aizpurua, J. J. Baumberg,
Threading plasmonic nanoparticle strings with light. Nat. Commun.
5, 4568 (2014). |
 |
| When twisted light matches the twist
of nanostructures, strong interactions with chiral molecules
could arise. |
|
A combination of nanotechnology and a unique twisting
property of light could lead to new methods for ensuring the purity and
safety of pharmaceuticals
16 May 2014 University
of Cambridge
A direct relationship between the way in which light is twisted by
nanoscale structures and the nonlinear way in which it interacts with matter
could be used to ensure greater purity for pharmaceuticals, allowing for "evil twins" of drugs to be identified with much greater sensitivity.
Researchers from the University of Cambridge have used this relationship,
in combination with powerful lasers and nanopatterned gold surfaces, to
propose a sensing mechanism that could be used to identify the right-handed
and left-handed versions of molecules.
Some molecules are symmetrical, so their mirror image is an exact copy.
However, most molecules in nature have a mirror image that differs - try
putting a left-handed glove on to your right hand and you'll see that your
hands are not transposable one onto the other. Molecules whose mirror-images
display this sort of "handedness" are known as chiral.
The chirality of a molecule affects how it interacts with its
surroundings, and different chiral forms of the same molecule can have
completely different effects. Perhaps the best-known instance of this is
Thalidomide, which was prescribed to pregnant women in the 1950s and 1960s.
One chiral form of Thalidomide worked as an effective treatment for morning
sickness in early pregnancy, while the other form, like an "evil twin",
prevented proper growth of the foetus. The drug that was prescribed to
patients however, was a mix of both forms, resulting in more than 10,000
children worldwide being born with serious birth defects, such as shortened
or missing limbs.
When developing new pharmaceuticals, identifying the correct chiral form
is crucial. Specific molecules bind to specific receptors, so ensuring the
correct chiral form is present determines the purity and effectiveness of
the end product. However, the difficulty with achieving chiral purity is
that usually both forms are synthesised in equal quantities.
Researchers from the University of Cambridge have designed a new type of
sensing mechanism, combining a unique twisting property of light with
frequency doubling to identify different chiral forms of molecules with
extremely high sensitivity, which could be useful in the development of new
drugs. The results are published in the journal Advanced Materials.
The sensing mechanism, designed by Dr Ventsislav Valev and Professor
Jeremy Baumberg from the Cavendish Laboratory, in collaboration with
colleagues from the UK and abroad, uses a nanopatterned gold surface in
combination with powerful lasers.
Currently, differing chiral forms of molecules are detected by using
beams of polarised light. The way in which the light is twisted by the
molecules results in chiroptical effects, which are typically very weak. By
using powerful lasers however, second harmonic generation (SHG) chiroptical
effects emerge, which are typically three orders of magnitude stronger. SHG
is a quantum mechanical process whereby two red photons can be annihilated
to create a blue photon, creating blue light from red.
Recently, another major step towards increasing chiroptical effects came
from the development of superchiral light - a super twisty form of light.
The researchers identified a direct link between the fundamental
equations for superchiral light and SHG, which would make even stronger
chiroptical effects possible. Combining superchiral light and SHG could
yield record-breaking effects, which would result in very high sensitivity
for measuring the chiral purity of drugs.
The researchers also used tiny gold structures, known as plasmonic
nanostructures, to focus the beams of light. Just as a glass lens can be
used to focus sunlight to a certain spot, these plasmonic nanostructures
concentrate incoming light into hotspots on their surface, where the optical
fields become huge. Due to the presence of optical field variations, it is
in these hotspots that superchiral light and SHG combine their effects.
"By using nanostructures, lasers and this unique twisting property of
light, we could selectively destroy the unwanted form of the molecule, while
leaving the desired form unaffected," said Dr Valev. "Together, these
technologies could help ensure that new drugs are safe and pure."
Full bibliographic information
V. K. Valev, J. J. Baumberg, B. De Clercq, N.
Braz, X. Zheng, E. J. Osley, S. Vandendriessche, M. Hojeij, C. Blejean, J.
Mertens, C. G. Biris, V. Volskiy, M. Ameloot, Y. Ekinci, G. A. E.
Vandenbosch, P. A. Warburton, V. V. Moshchalkov, N. C. Panoiu, T. Verbiest,
Nonlinear superchiral meta-surfaces: tuning chirality and disentangling
non-reciprocity at the nanoscale. Adv. Mater. 26, 4074-4081 (2014). |
 |
| Shining circularly polarised light on
ring-shaped nanostructures increases the opportunity for
interaction with molecules. |
|
"Loops of light" promising for optical detection of
individual molecules
18 July 2012 KU Leuven University
KU Leuven researcher Ventsislav Valev and an international team of
colleagues have developed a new method for manipulating light at the
nanoscale in order to optically detect single molecules. By shining
circularly polarised light on a gold, square-ring shaped nanostructure, the
researchers were able to "activate" the entire surface of the nanostructure,
thereby significantly increasing the opportunity for interaction with
molecules. The method has a broad range of potential applications in nanoscale photochemistry and could assist in the advancement of technologies
for visualising single molecules and multiple-molecule interactions.
Nanotechnology researchers around the world are exploring ways to
optically detect single molecules, but progress can be hindered by the fact
that single molecules have extremely weak optical responses. Thus far,
scientists have developed a way to use metal nanostructures to focus light
into tiny spots called "hotspots". The hotspots excite electrons on the
surface of the nanostructure, causing them to oscillate coherently. When
shone on a molecule, and with the help of these oscillating electrons, the
focused light can increase a molecule's optical signal to 100 billion times
its normal strength. This signal can then be detected with an optical
microscope.
But there are two limitations to the current method: hotspots can become
too hot, and they are just spots. That is, the heat from hotspots can melt
the nanostructures, thus destroying their ability to channel light
effectively, and hotspots produce only a very small cross-section in which
interaction with molecules can take place. Additionally, for a single
molecule to become detectable, it needs to find the hotspot.
Loops of light
In order to overcome these limitations, Dr. Valev and his colleagues
sought out to nanoengineer larger spots. They began by shining
circularly polarised light rather than linearly polarised light on the
nanostructures and found that this could increase the useful area of these
nanostructures. More importantly, when shone on square-ring shaped gold
nanostructures, the scientists observed that the entire surface of
the nanostructures was successfully activated.
Dr. Valev explains: "Essentially, light is constituted of electric and
magnetic fields moving through space. While with linearly polarised light,
the fields move in a linear, forward direction, with circularly polarised
light, they rotate in a spiral-like motion." The circularly polarised light
imparts a sense of rotation on the electron density in ring-shaped gold
nanostructures, thus trapping the light in the rings and forming "loops of
light". The loops of light cause excited electrons to oscillate coherently
on the full surface of the square-ringed nanostructures - rather than in a
few concentrated hotspots. This increases the opportunity for interaction
with molecules: "The trick is to try to activate the whole surface of the
nanostructure so that whenever a molecule attaches, we will be able to see
it," says Dr. Valev. "That is precisely what we did."
The method has a broad range of potential applications in nanoscale
photochemistry and could assist in the advancement of technologies for
visualising single molecules and multiple-molecule interactions. The
findings were published in the scientific journal Advanced Materials.
Full bibliographic information
V. K. Valev, B. De Clercq, C. G. Biris, X. Zheng, S.
Vandendriessche, M. Hojeij, D. Denkova, Y. Jeyaram, N. C. Panoiu, Y. Ekinci,
A. V. Silhanek, V. Volskiy, G. A. E.
Vandenbosch, M. Ameloot, V. V. Moshchalkov, and
T. Verbiest, Distributing the Optical
Near-Field for Efficient Field-Enhancements in Nanostructures. Adv. Mater. 24, OP208-OP215, (2012). |
 |
| Similar
to the way water backjets eject droplets of water on the surface
of a pond, powerful laser pulses can locally melt gold
nanostructures and produce gold nanojets, ejecting perfectly
spherical gold nanodroplets. |
|
Perfectly spherical gold nanodroplets produced with the
smallest-ever nanojets
13 January 2012
KU Leuven University
KU Leuven researcher Ventsislav Valev and an international team of
scientists have developed a new method for optical manipulation of matter at
the nanoscale. Using "plasmonic hotspots" - regions with electric current
that heat up very locally - gold nanostructures can be melted and made to
produce the smallest nanojets ever observed. The tiny gold nanodroplets
formed in the nanojets, are perfectly spherical, which makes them
interesting for applications in medicine.
The "backjet" phenomenon on which the method turns can be compared to a
pebble being dropped into water. Tightly focused ultrafast laser pulses
carry sufficient energy to locally melt the surface of a gold film. When a
laser pulse of light hits the film, a nanoscale backjet - a nanojet - of
molten gold surges upward.
As the name suggests, nanojets on the surface of a homogeneous gold film
are incredibly small, their size being determined by the distribution of
energy in the light pulse. This distribution of energy is in turn dependent
on the wavelength of light. Initially, scientists anticipated that nanojets
could not be significantly smaller than the wavelength of light. In this
study however, Ventsislav Valev and his colleagues show that nanojets can in
fact be made much smaller with the help of "plasmonic hotspots".
Plasmonic hotspots are regions on the surface of metal nanostructures
where light causes very strong oscillation of the electrons. Because
electron oscillations constitute an electric current and because electric
currents heat up the material the same way an electric stove heats up in the
kitchen, the plasmonic hotspots are extremely hot. So hot that they can melt
the gold in a spot much smaller than the wavelength of light. Dr. Valev and
his colleagues were successfully able to demonstrate that this tiny little
pool of molten gold can give rise to the smallest nanojets ever observed.
The gold nanodroplets propelled upward by the nanojets solidify in
flight, producing perfectly spherical nanoparticles. These gold nanodroplets
can be collected and used for medical applications including cancer
treatment. The nanoparticles can be attached to molecules and injected in
the blood. Once the molecules attach to cancer cells, light can be used to
heat up the gold nanodroplets and destroy the cancer cells. Currently, the
gold nanoparticles used in medications are chemically synthesised. These
chemically synthesised gold nanoparticles have an unavoidably granular
aspect. Conversely, gold nanodroplets created by the plasmonic nanojet
method detailed by Dr. Valev and his colleagues are perfectly spherical,
ensuring a better efficiency.
Full bibliographic information
V. K. Valev, D. Denkova, X. Zheng, A. I. Kuznetsov, C. Reinhardt, B. N.
Chichkov, G. Tsutsumanova, E.J. Osley, V. Petkov, B. De Clercq, A. V.
Silhanek, Y. Jeyaram, V. Volskiy, P. A. Warburton, G. A. E. Vandenbosch, S.
Russev, O. A. Aktsipetrov, M. Ameloot, V. V. Moshchalkov, T. Verbiest,
Plasmon-enhanced sub-wavelength laser ablation: plasmonic nanojets.
Adv. Mater. 24, OP29-OP35 (2012). |
 |
| Surface plasmon patterns can be
imprinted on metallic nanostructures for subsequent high
resolution imaging with standard surface probe techniques.
|
|
Imaging of surface plasmons may be a lot easier than you
thought
06 June 2011
KU Leuven University
An unusual observation turned into a scientific breakthrough when KU
Leuven researchers investigating the optical properties of nanomaterials
discovered that so-called surface plasmons leave imprints on the surface of
the nanostructures. This leads to a new type of high resolution microscopy
for imaging the electric fields of nanostructures.
Nanomaterials, consisting of extremely small particles or thin layers,
tend to acquire unexpected properties. Optical nanomaterials are a class of
materials that have emerged over the last ten years and that have quickly
become a hot topic in material science due to their counterintuitive optical
behavior and revolutionary potential applications. Optical nanomaterials are
mainly based on surface plasmon resonances - the property whereby, in
metallic nanostructures, light can collectively excite surface electron
waves. These electron waves have the same frequency as light, but much
shorter wavelengths, which allow their manipulation at the nanoscale. In
other words, with the help of plasmons, light can be captured, modified and
even stored in nanostructures. This emerging technology finds applications
in surprising areas, ranging from cancer treatment (by targeting cancer
cells with nanoparticles that will produce heat when excited) to
invisibility (by causing light to follow a trail of nanoparticles, that acts
as an invisibility cloak to whatever is underneath them).
The imaging of surface plasmons provides a direct way to map and
understand the local electric fields that are responsible for the unusual
electromagnetic properties of optical nanomaterials. However, the imaging of
surface plasmons is quite challenging. While there are methods to image
plasmons with high resolution, they come at a considerable increase in both
cost and complexity. But now, Ventsislav K. Valev and his colleagues have
demonstrated a powerful and user friendly method for imaging plasmonic
patterns in nanostructures.
"We were performing routine characterization of freshly grown samples,
when I asked Yogesh, one of our Ph.D. students, to look at a sample that had
already been studied. There was absolutely no reason to do this; I just had
a hunch," sais Ventsislav Valev. "Surprisingly, this sample appeared to be
decorated and I immediately recognized the pattern. Somehow, the optical
properties have been imprinted on the surface of the nanostructures."
The scientists indeed found out that upon illuminating nanostructures
made of nickel or palladium, the resulting surface plasmon pattern is
imprinted on the structures themselves. This imprinting is done through
displacing material from the nanostructure to the regions where the plasmon
enhancements are the largest. In this manner, the plasmons are effectively
decorated, allowing for subsequent imaging with standard surface probe
techniques, such as scanning electron microscopy or atomic force microscopy.
The imprinting method is quite unique, combining aspects of both imaging and
writing techniques.
This research is described in an upcoming paper in the journal Physical
Review Letters.
Full bibliographic information
V. K. Valev, A. V. Silhanek, Y.
Jeyaram, D. Denkova, B. De Clercq, V. Petkov, X. Zheng, V. Volskiy, W.
Gillijns, G. A. E. Vandenbosch, O. A. Aktsipetrov, M. Ameloot, V. V.
Moshchalkov, and T. Verbiest, Hotspot
Decorations map plasmonic patterns with the resolution of scanning probe
techniques.
Phys. Rev. Lett. 106, 226803 (2011). |
©
www.valev.org |