Some miscellaneous comments on adhesion
D. E. Packham,
Materials Research
Centre,
University of Bath.
For a collection of short, self-contained articles,
which together give comprehensive account
of the scientific, engineering and industrial aspects of adhesion, see:
D.E. Packham (ed.), Handbook of Adhesion, 2nd edition, Wiley, 2005.
available on line:
http://www3.interscience.wiley.com/cgi-bin/bookhome/111084161
However, some miscellaneous comments on adhesion are provided below
Bonds at the interface
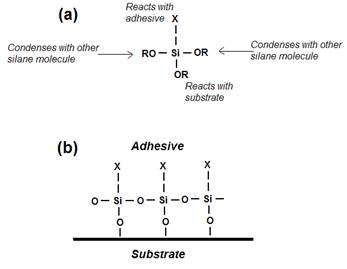
Organo-silane
adhesion promoters are widely used to enhance the adhesion and bond
durability with glass and metals. Typical structure (a) and postulated mode of
action (b) for a silane adhesion promoter. There is much evidence that their
mode of action is much more complex than this, e.g. see discussion in
D.E. Packham, Some contributions
of surface analysis to the development of adhesion theories, J. Adhesion 84,240–255(2008).
The next figure shows various interactions postulated
between PMMA and surfaces of differing
acidic and basic nature. It is based on work of
S.R. Leadley, and J.F. Watts, J. Adhesion 60(1-4) 175-196 (1997).
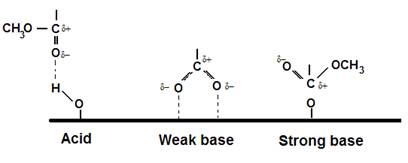
E.G. (Si) (Al)
(Ni)
H-bond (Si) ionic
(Al)* ion/dipole (Ni)
* indicating
hydrolysis of the ester and adsorption through the carboxylate anion
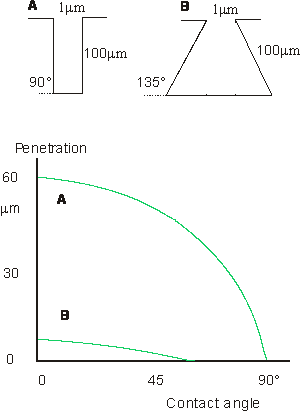
Rough surfaces
With rough
surfaces there may be serious problems of the adhesive's being abl to enter
proes and crevices on the substrate surface. This figure shows equilibrium penetration of a liquid into
cylindrical and "ink bottle" pores, after de Bruyne.
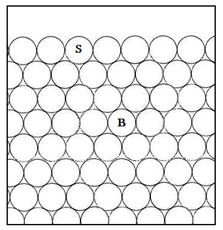
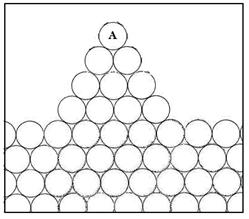
A rough surface will have a higher surface energy than a corresponding smooth one. This is
shown in the schematic diagram below. In this simplified two dimensional representation,
the bulk atom (B) has 6 nearest neighbours, the atom on a plane surface (S) has
4, and that on an asperity on a rough surface (A) only 2.
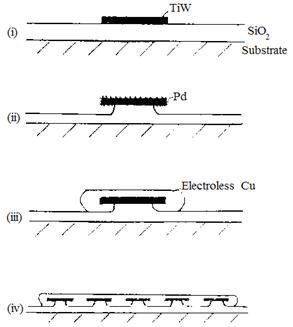
Some macroscopically rough
surfaces are known to enhance adhesion.
Here undercut TiW islands enhance adhesion of copper to silica. Based on
A.M.T. van der Putten, J. Electrochem.
Soc. 140, 2376(1993). (i) TiW
islands deposited (ii) Pd activator adsorbed and HF etching; (iii) electroless
Cu deposited; (iv) Cu electrodeposited.
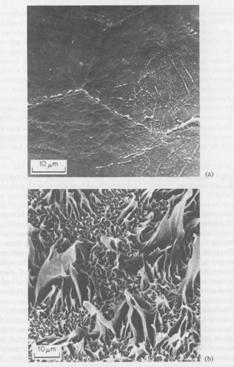
Some very rough surfaces can result from pretreatment prior
to adhesive bonding. Here are some microfibrous
surfaces (l to r): dendrites of zinc electrodeposited onto a zinc surface;
black CuO layer produced on copper; PTFE irradiated by argon ions. The last
based on S.K. Koh, S.C. Park, S.R. Kim, W.K. Choi, H.J. Jung, and K.D. Pae, J. Appl. Polym. Sci., 64,1913(1997), courtesy of the authors.
The increased
adhesion to microfibrous surfaces is often associated with increased
plastic deformation of the polymer during failure of the adhesive bond, e.g. substrate
surface after peeling LDPE from (a) polished copper and (b) copper with a microfibrous oxide surface:
The concentration of stress at the "fibre" tips
can be demonstrated using a photoelastic model [J.R.G. Evans, Ph.D. thesis,
University of Bath 1977]:

Polymer-polymer bonding
Bonds between
immiscible polymers (A & B) are usually very weak, but some interdiffusion
can occur if the interaction parameter is not too high, cf. R.P. Wool: Polymer interfaces: structure and strength,
Hanser, Munich (1995) & in Adhesion
Science and Engineering, Vol. II, Surfaces,
Chemistry and Applications, Assoc. Ed. Chaudhury, Manoj K. and Pocius, A.V.
Elsevier, p. 351-402, 2002
The figure shows how
the laminating temperature affects the adhesion of four different ethylene-octene
copolymers to polypropylene (ethylene-octene copolymers and polypropylene are
formally incompatible):
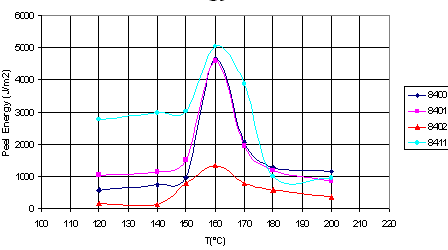
L. Godail and D.E.
Packham, J. Adhesion Sci. Technol. 15(11), 1305(2001).
The interface between
immiscible polymers (A & B) may be strengthened by introduction of a diblock
copolymer AB. Here the A-block length is below entanglement length, so the
overall toughening is modest.
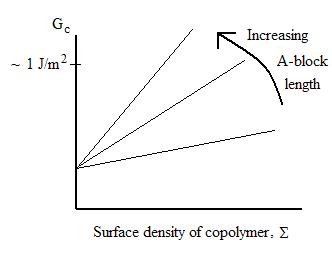
However, with a long chain length diblock copolymer, much
higher toughness is obtainable:
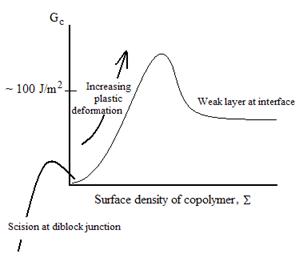
Results like these have been published by Creton and
colleagues, among others:
C. Creton, H.R. Brown, and V.R.
Deline, Macromolecules 27,
1774(1994).
C. Creton, H.R. Brown, and K.R.
Shull, Macromolecules 27, 3174(1994).
Random copolymers
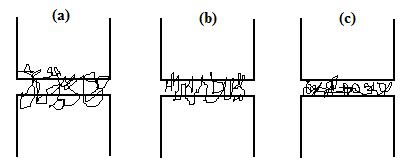
– usually cheaper than diblocks – can also be used if the incompatibility
between the homopolymers is not too large. The molecule of a random copolymer
will form coils wandering many times across interface, forming many
"stiches". For large incompatibility the copolymer will simply form a
collapsed globules at interface, giving no enhancement of adhesion. Incompatibility
increases in the order (a), (b), (c):