Modelling Real Surface Structures
The structure and stability of solid surfaces are key factors that control a wide range of technologically important processes, including sintering, catalysis and corrosion.
Energy Screening - Hematite
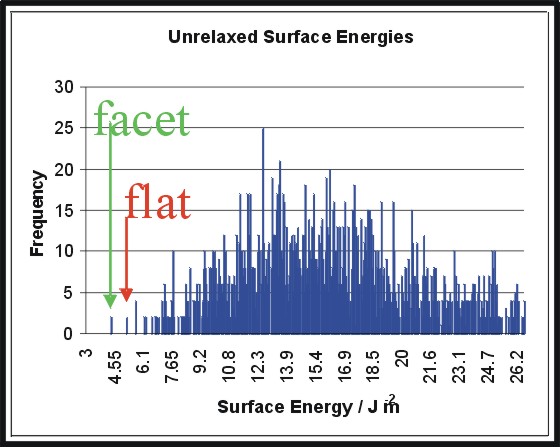
In nature surfaces are not totally flat and defect free. Therefore we must sample all possible configurations. In this example we consider the Hematite (01.1) surface and decribe a process called energy screening.
The hematite (01.1) unit cell consists of 20 ions. There are 2 20 possible configurations. By considering non dipolar surfaces and symmetry this is reduced to give 1277 reasonable configurations.
Each surface configuration is relaxed. We find that many of
the most stable surfaces are facetted, for different materials.
|
Flat, 3.0 J/m2 |
Facet, 2.6 J/m2 |
 |
Microfaceting on the {110} surfaces of MgO
The following figure illustrates that the pefectly cleaved {110} surface of MgO is less stable (a higher surface energy) than the microfaceted surface. Thus we can predict that the {110} surface of MgO crystals could be comprised of {100} facets.
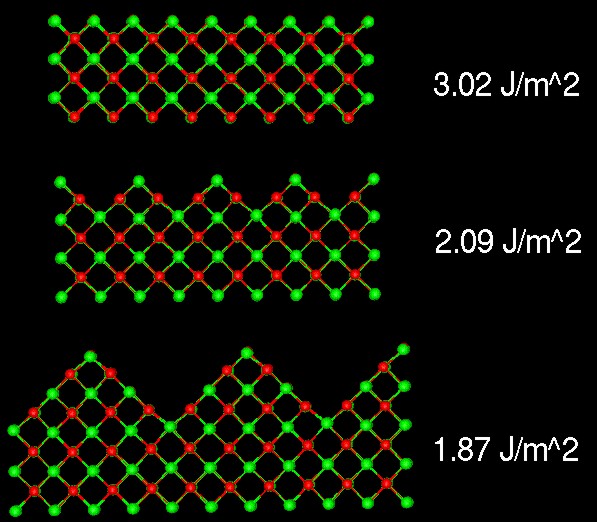
Watson G.W., Kelsey E.T., de Leeuw N.H., Harris D.J. and Parker S.C. 'Atomistic simulation of dislocations, surfaces and interfaces in MgO' J. Chem. Soc., Faraday Trans., 92, 433-438 (1996)
Hydration energies for the {110} surface of MgO
The hydration energy for the faceted {110} surface of MgO was calculated as a function of coverage. The energy is essentially constant showing classic Langmuir behaviour.
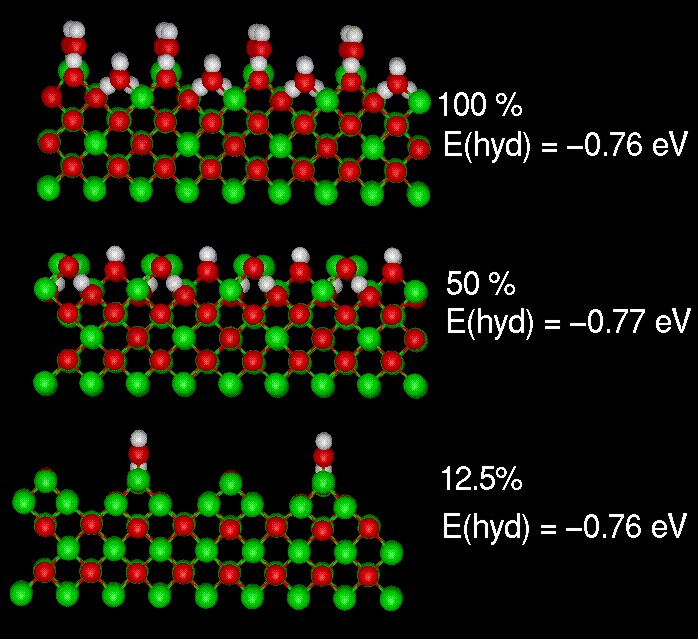
de Leeuw N.H., Watson G.W. and Parker S.C. 'Atomistic simulation of the effect of dissociative adsorption of water on the surface structure and stability of calcium and magnesium oxide' J. Phys. Chem 99, 17219-17225 (1995)
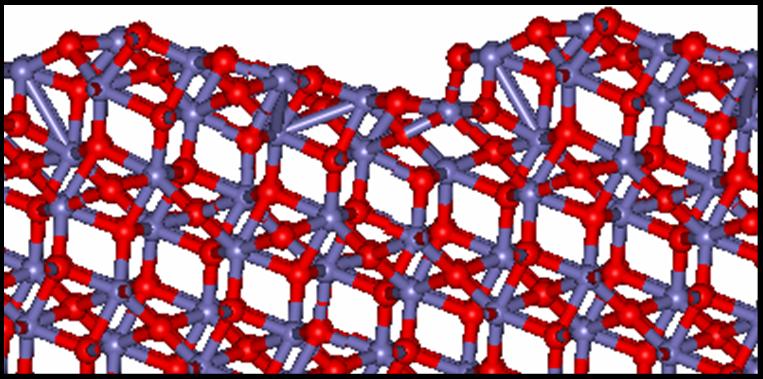
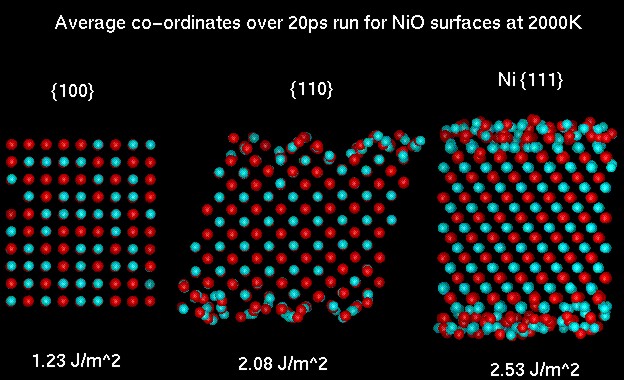
Molecular dynamics simulation of the {100}, {110} and Ni {111} surface of NiO at 2000K
The average co-ordinates for the 20ps data collecting run for {110} and Ni {111} slabs indicate that there is considerable disorder at the surface. This is especially evident for the Ni {111} surface where the diffusion constant for the ions in the top most layers approaches that of a liquid at this temperature. An interesting result from the {110} simulation is that even when the flat surface is modelled it rearranges to form a facet structure similar to that described above for MgO.
Oliver P.M., Watson G.W. and Parker S.C. 'Molecular dynamics simulations of nickel oxide surfaces' Phys. Rev. B 52, 5323-5329 (1995)