A Novel Extractive Membrane Bioreactor For Treating Bio-Refractory Organic Pollutants In The Presence Of High Concentrations Of Inorganics: Application To a Synthetic Acidic Effluent Containing High Concentrations Of Chlorophenol And Salt.
W. Liu 1, J.A. Howell 1, T.C. Arnot 1* and J.A. Scott 2
1
Department of Chemical Engineering, University of Bath, Claverton Down, Bath, BA2 7AY, UKEmail: T.C.Arnot@bath.ac.uk or J.A.Howell@bath.ac.uk
2
Centre for Integrated Environmental Protection, Nathan Campus, Griffith University, Brisbane, Queensland 4111, AustraliaEmail: J.A.Scott@mailbox.gu.edu.au
* corresponding author
Abstract
Traditional bioprocesses have difficulties treating bio-refractory organic pollutants in waste streams that contain high concentrations of inorganics. A novel hybrid process, combining liquid-liquid extraction and a membrane bioreactor, has been designed to treat such an effluent. To demonstrate the viability of this process, a synthetic wastewater was designed to represent the sort of effluent that often results from the speciality organic chemicals sector. This model effluent contains high concentrations of chlorophenols (CPs) (1,000 mg.l-1), salts (5 % w/w) and has a pH of less than 1. Experimental work was combined with process design to estimate the likely treatment costs for such an effluent. The proposed treatment process has three advantages:
This process could also be used for treating other wastewaters containing organic priority pollutants that are either acids or bases.
Keywords: Membrane bioreactor; Water treatment; Priority pollutants; Extraction; Filtration
1. Introduction
Modern industry exploits various synthetic organic compounds, which are bio-refractory, bio-accumulatory and/or carcinogenic. 129 priority pollutants have been listed by the US Environmental Protection Agency [1] and by the European Economic Community [2]. Over the last decade a variety of microbial species have been identified as being able to biodegrade many of these xenobiotic compounds [3-7]. Among the 129 priority pollutants, 41 are easily biodegradable and another 29 can be biodegraded by specific bacterial strains under controlled conditions [8]. Where it is possible biodegradation is the most economical method for effluent treatment, particularly in comparison with incineration, which is the common and far more expensive alternative.
Industrial waste streams containing high concentrations of biorefractories should ideally be treated at source. If such streams are mixed and diluted with other wastewater, which contains easily biodegradable substrates, before being discharged to sewer, bacteria in a municipal treatment plant will preferentially metabolise the latter and the toxic contaminants will pass through unaltered as ‘Hard COD’ [9]. Unfortunately, many of the concentrated streams from say organic chemical synthesis, exhibit extremes of pH, high concentrations of salts (up to 20 % w/v), and carried-over catalysts, one or all of which may inhibit microbial growth. Table 1 lists a number of biorefractory organics which often occur in this type of effluent, and which would be amenable to treatment using this process. One of them, chlorophenol (CP), which is manufactured for use as an intermediate in chemical syntheses of agrochemicals, dyestuffs and pharmaceuticals, or for direct use in formulations has been selected as an example. Effluents from the production of these types of compound typically contain a high concentration of salt, as well as some residual levels of organic, or chlorinated organic, compounds [10]. The precise composition of specific effluent streams is commercially sensitive information, so to demonstrate the potential of this hybrid effluent treatment process we propose a synthetic effluent to be representative of this type of effluent material:
As a priority pollutant, the concentration of 2,4-DCP in effluents discharged to the environment is very strictly regulated in the discharge standards of the USA [11] and the European Community [12] at 200 mg.l-1 for a daily maximum and 100 mg.l-1 as a 4-day rolling average. A conceptual multi-unit process of liquid-liquid extraction and membrane bioreactor – the extraction-membrane bioreactor (EMBR) is outlined for treating such streams. It may be generic for a sub-class of mixed organic/inorganic industrial effluents in which the priority pollutant is acidic or basic. Experiment results on each process unit are reported and other factors related to the overall process are discussed.
2. Theory
The diagram of the conceptual extraction membrane bioreactor (EMBR) process is shown in Figure 1. There are 4 units in the process:
2.1 Extraction unit
The acidic organic pollutants in the acidic wastewater (Stream a in Figure 1: 1,000 mg.l-1 2,4-DCP, 5% (w/w) sodium chloride, pH < 1) are extracted by a water insoluble organic solvent. The raffinate is discharged after filtering through a hydrophilic microfiltration membrane that minimises loss of organics in droplet form (Stream b in Figure 1: <100 mg.l-1 2,4-DCP, 5% (w/w) sodium chloride, pH < 1).
2.2 Stripping unit
The extract is pumped from the extraction unit to the stripping unit where organic pollutants are transferred into an alkaline aqueous phase free of concentrated inorganics. The pH shift between the aqueous phases in the extraction and stripping units is vitally important. For example, 2,4-DCP is quickly stripped from the organic phase into the aqueous phase by adding sodium hydroxide. 2,4-DCP easily dissociates:
![]() ()
()
in alkaline conditions and its concentration in the raffinate out of the stripping unit (Stream c in Figure 1: o/w emulsion, free of sodium chloride, pH » 12) is much higher than that in the treated water (Stream b in Figure 1). The raffinate is now salt free.
2.3 Membrane o/w emulsion separation and biomass separation
Organic droplets in the raffinate from the stripping unit (Stream c in Figure 1) are filtered out by the hydrophilic ceramic membrane, thus the organic pollutants are fed into the bioreactor but not the solvent droplets. The same membrane also serves to hold bacteria in the bioreactor. The outlet stream of the bioreactor is filtered from the reverse direction in a second membrane. These units are periodically switched in function. After the switch the filtration of biomass separation serves to backflush the membrane previously used for filtering the o/w emulsion and vice versa. In this way, membrane fouling is reduced.
2.4 Bioreactor
The organic pollutants are fed to the bioreactor as the carbon and energy sources for the bacteria and may be supplied to be present at optimum concentrations, suitable pH and temperature. This optimum concentration for biodegradation being much higher than the discharge consent restraint for the pollutant in the treated effluent allows a much smaller bioreactor than would be needed for straight biodegradation of the pollutant in the original effluent feedstream. When the pollutants are chlorinated hydrocarbons, hydrochloric acid is released as the biodegradation by-product. The hydroxide ion carried by the inlet stream is consumed to neutralise the hydrochloric acid. The pH in the bioreactor is controlled by the alkali feed to the aqueous phase in the stripper serving to maintain the bioreactor at neutral pH and the stripper at alkaline pH.
2.5 Generic nature of the process
This EMBR process could be used for treating many industrial waste streams containing various hazardous compounds. It is useful where there can be a pH shift between waste and bioreactor and where the particular pollutant of interest is acidic or basic. This allows the pH control of the bioreactor to provide the pH shift across the exctraction-stripping unit which gives the higher pollutant concentration in the bioreactor. The waste streams containing a high concentration of 2,4-DCP and sodium chloride are taken as a specific example and experimental results are reported.
3. Materials and methods
3.1 Chemicals
2,4-DCP, acetic anhydride, toluene and heptane were supplied by the Aldrich Chemical Company UK. pH buffers, potassium hydroxide, phosphoric acid, potassium carbonate, ammonium sulphate, magnesium sulphate [MgSO4.7H2O], sodium nitrate, calcium nitrate [Ca(NO3)2.4H2O] and ferrous sulphate [FeSO4.7H2O] were supplied by Fisher Scientific Ltd. UK., and nutrient agar and yeast extract by Oxoid Ltd. UK.
3.2 Membranes
Fairey Industrial Ceramics Ltd. UK supplied the hydrophilic corundum (a-Al2O3) membranes as 600 mm long, 20 mm diameter microfiltration (MF) modules (pore size 0.35 mm), with seven internal star-shaped channels of approximately 2 mm diameter. The membrane is not swollen by solvents and can withstand a wide pH range (0.5 to 13.5). It is also amenable to backflush operation. The membrane area for forward filtration (feed on the channel side) was 0.06 m2 and reverse filtration (feed on the outer side) 0.038 m2.
3.3 Analytical techniques
The 2,4-DCP concentration in the aqueous phase was measured by gas chromatography after acetylation and toluene extraction. This method is much simpler than Method 604 proposed by US EPA [13] and has a detection limit of 10 µg.l-1 2,4-DCP, which is sensitive enough for this research. The 2,4-DCP concentration in the aqueous phase was measured as the following: 1.7 g potassium carbonate and 0.8 ml acetic anhydride were added into 100 ml of aqueous sample. The pH of the aqueous solution was adjusted to 8.5 ~ 9 by adding more potassium carbonate. 2,4-DCP was acetylated to form acetyl 2,4-DCP. The acetylation reaction took 10 minutes.
The acetylated sample was then extracted with 5 ml of toluene. The toluene layer was separated by a centrifuge (P Selecta) at 4,000 rpm for 4 minutes. 1 ml of the toluene solution was automatically injected by a HP7673 auto-injector into HP 5890 II GC with an electron capture detector (ECD) and a BPX5 column, 50 m long and 0.22 mm id, (SGE Ltd. UK.). The oven temperature was kept at 160 °C, injection temperature 225 °C and detector temperature 350 °C. The helium carrier gas flow rate was kept at 1 ml.min-1 and total gas flow rate (helium and nitrogen) at 60 ml.min-1 (10 % purge and 90 % makeup gas). The retention time of acetyl 2,4-DCP was 10.8 minutes and the peak area was integrated by a Delta instrument (SGE Australia Ltd.). The concentration was then calculated according to a calibration curve.
The 2,4-DCP concentration in the diethyl sebacate solution was measured using the same GC system in a similar way. In order to separate 2,4-DCP from diethyl sebacate, a solvent with a boiling point of 312 °C, 0.5 µl of the sample was introduced as liquid directly onto the GC column using cool on-column technique. The diethyl sebacate in the o/w emulsion was first extracted by heptane before GC analysis. The oven temperature ramped from 150 °C to 312 °C at 2 °C per minute, the injection temperature was raised accordingly from 153 °C to 315 °C at 2 °C per minute. The retention time of 2,4-DCP was 4.5 minutes.
Total organic carbon (TOC) concentration was measured using a DC-180 Carbon analyser with infrared detector (Rosemount Ltd. UK.), and all other methods and procedures are fully described by Liu [14].
4. Results and discussion
4.1 Extraction unit
It may be economically acceptable to remove toxic contaminants from concentrated wastewater by liquid-liquid extraction and then recover or destroy them [15, 16]. The choice of a suitable solvent is the first and basic consideration for the development of the EMBR process. The solvent used in biological wastewater treatment process needs to have
4.1.1 Partition Coefficient
For treating the specific wastewater containing 2,4-DCP and sodium chloride, diethyl sebacate [C2H5O2C(CH2)8CO2C2H5] or DES was considered to be a suitable solvent. The target DCP distributes favourably into DES with a measured partition coefficient varying from 103.23 to 102.85 when the 2,4-DCP concentration in the aqueous phase is 17.1 to 1,620 mg.l-1 and the pH of the aqueous phase is 2.1 to 2.3.
No sodium chloride was added into the aqueous solution during the experiment to simulate the real wastewater, which actually contains about 5% (w/w) of sodium chloride. It was reported [21] that the distribution coefficients of 1-butanol and acetone in various solvent (Adol 85NF, n-valeradehyde, tert-amyl alcohol and cyclopentanol) and water systems, were markedly increased after adding salts, and the effects of sodium chloride was stronger than that of potassium acetate. With increasing concentration of electrolytes in the aqueous solution, the activity coefficients of non-electrolytes in the solution increase [22]. The lower pH of the effluent would also enhance the partition. The UNIFAC model [20] estimated the partition coefficient to be 7,000.
4.1.2 Solvent loss
UNIFAC estimated the solubility of diethyl sebacate in water to be 200 mg.l-1. Experimentally it was not found possible to detect diethyl sebacate in water after 48 hours extraction followed by 24 hours settling. Even lower solubility would be expected in the presence of salt at high concentration. Losses are likely to be negligible in the treated water.
4.1.3 Chemical and thermal stability
50 ml of diethyl sebacate was well mixed with 100 ml 2,4-DCP water solution at pH 2.3. It was then kept at 25 ° C and shaken at 250 rpm for two months, no visible loss of diethyl sebacate was detected.
4.1.4 Biocompatibility
The toxicity of a solvent to bacteria can be separated into phase toxicity and dissolved toxicity. In the EMBR process, the solvent droplets are isolated outside the bioreactor by the hydrophilic membrane, so that the phase toxicity can be excluded and only the dissolved toxicity needs to be considered. In general, the solvent tolerance of Gram-negative bacteria is higher than that of Gram-positive bacteria [23]. Comparing with many other bacteria, P. putida, P. chlororaphis and P. syringae, have the highest solvent tolerance [24] and they can biodegrade CPs. Diethyl sebacate was successfully used in a two-phase extractive bioreactor to enhance the biodegradation of penta-chlorophenol by Arthrobactor sp. [25]. The experiment in this research showed that diethyl sebacate is very biocompatible and tends to be rather biodegradable.
4.1.5 Non-biodegradability
The normal extractive fermentation process requires a non-biodegradable solvent otherwise the system is uneconomical. According the authors’ observation, diethyl sebacate is biodegradable. This is different from the result of Daugulis et al. [25] who found diethyl sebacate to be non-degradable. Fortunately, in the EMBR process, the diethyl sebacate droplet is prevented from entering the bioreactor by the hydrophilic membrane and its solubility in water is low and so biodegradability is not an issue.
4.2.1 Extraction unit
A mixer/settler was selected for the extraction unit, because it can offer a high stage efficiency [26]. In order to reach a very low 2,4-DCP concentration of 100 mg.l-1, at least two extraction stages are necessary. A detailed extractor design is beyond the scope of this paper. A dual membrane system is also possible but was not tested.
4.2.2 Stripping unit
The 2,4-DCP in the diethyl sebacate is stripped into an alkaline aqueous phase. The extracted solvent out of the stripping unit should have a low 2,4-DCP concentration, thus diethyl sebacate can be recycled to the extraction unit to extract more 2,4-DCP. The raffinate of the stripping unit should have a high total concentration of 2,4-DCP and its anion (2,4-DCP-), to maintain an optimum 2,4-DCP concentration in the bioreactor.
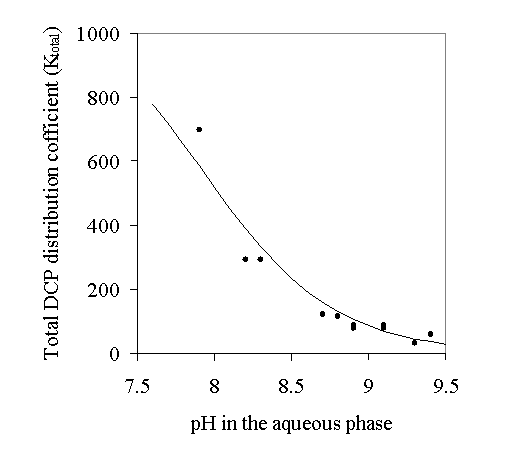
The distribution coefficients between diethyl sebacate and water at different pH were measured using the same technique as before and are shown in Figure 2.
2,4-DCP is an organic acid and it can dissociate in aqueous solution:
![]() ()
()
where:
![]() ()
()
The result of the 2,4-DCP extraction experiment showed that the distribution coefficient of 2,4-DCP between the diethyl sebacate and water is a constant:
![]() ()
()
If the 2,4-DCP anion (2,4-DCP-) is completely insoluble in diethyl sebacate, then the overall 2,4-DCP and 2,4-DCP- distribution coefficient between diethyl sebacate and water is:
![]() ()
()
Figure 3 shows that the experimental data fits well with Equation 5, and verifies that the solubility of the 2,4-DCP anion in diethyl sebacate is negligible. Because the readily available buffer in the market was pH = 10.00, the experiment was carried out at pH = 7.7 ~ 9.4, which is in the range of 2,4-DCP dissociation. In the stripping unit of the EMBR process, the pH can actually be around 12. At pH 12 K is estimated by Equation 5 to be 0.098
In 2,4-DCP extraction, the aqueous phase boundary layer resistance is dominant, but in 2,4-DCP stripping the case is different. A chemical reaction is taking place in the alkaline aqueous phase at a reaction plane located in the boundary layer:
![]() ()
()
As the pH increases the degree of ionisation increases thus diminishing the aqueous phase boundary layer mass transfer resistance. Detailed stripper design is beyond our discussion.
4.3 O/w emulsion membrane filtration
Several investigators have demonstrated over 99.9% rejection of oil by microfiltration and ultrafiltration of the o/w emulsion independent of membrane type and oil concentration. All permeate oil concentrations were less than 20 mg.l-1 [27]. The hydrophilic ceramic membrane gave better performance than polymeric membranes [28-30].
When o/w emulsion is separated by hydrophilic membrane filtration, water passes through the membrane and the big oil droplets are retained on the membrane surface by a sieving mechanism. The deformable oil droplets cannot be squeezed into pores unless the applied pressure is higher than the capillary pressures, which can be expressed as:
![]() ()
()
where: ![]() is the interfacial tension between oil and water,
is the interfacial tension between oil and water,
![]() is the contact angle of an oil droplet on the membrane surface in the presence of water, and
is the contact angle of an oil droplet on the membrane surface in the presence of water, and
r
is the radius of the membrane pore.A schematic diagram of the device used for the following experiment is shown in Figure 3. It was designed to simulate the forward and the reverse filtration in the EMBR process. The o/w emulsion filtration was the reverse filtration in this experiment.
2% (w/w) diethyl sebacate/water emulsion and the permeate were stored in Vessels I and II respectively. Both liquids were well mixed and circulated by centrifugal pumps in cross-flow along different sides of one membrane module (A or B). Four solenoid valves S1, S2, S3 and S4 altered the emulsion and permeate flow directions and controlled the membrane filtration and backflush. When S1 and S4 were closed and S2 and S3 are opened, Module A operates as reverse filtration and Module B as forward filtration. The diethyl sebacate/water emulsion in Vessel I was filtered through Module A, entered Vessel II (the bioreactor in the EMBR process) and then went back to Vessel I, through Module B (biomass filtration in the EMBR process). When S1 and S4 were opened and S2 and S3 closed, both membrane modules were backflushed. The backflush length was set at 2 seconds. After 5 hours the functions of Modules A and B were switched, providing membrane cleaning. A computer controlled the whole device. Before each run, the complete system including the membrane modules was cleaned with Tergazyme solutions at 50 ° C, then with 1% ethanol distilled water solution and finally with distilled water. Each run lasted 15 hours to make sure that the diethyl sebacate concentration in the permeate side reached a constant value.
Permeate diethyl sebacate concentrations are summarised in Table 2 for different filtration conditions. The results of this study showed that the ceramic hydrophilic MF membrane could effectively separate the diethyl sebacate/water emulsion. Diethyl sebacate concentrations in the permeate were lowest when the TMP was low and when the membrane was frequently backflushed.
The size of membrane pores are not uniform but show a certain distribution. As pressure increases, oil droplets can be squeezed into more pores. This is why the permeate diethyl sebacate concentration was slowly increased with an increase in TMP.
Membrane fouling during o/w emulsion filtration is mainly due to adsorption of oil on membrane structure, which causes modification of the critical surface tension. The more serious the membrane fouling is, the more easily the emulsified oil droplets enter the membrane structure and the worse the separation will be [31]. Frequent backflushing was successfully applied to reduce the membrane fouling [30]. This study showed that frequent backflush also improved the diethyl sebacate separation. Very low diethyl sebacate permeate concentrations were realised when the membrane was backflushed once per 5 or 10 seconds. This can be explained as during the experiment, the average filtration flux under 0.1 bar TMP was about 50 l.m-2.hr-1 and the diethyl sebacate concentration in the o/w emulsion was 2% (w/w). If the initial 5 seconds of the cross-flow filtration is considered as dead-end filtration, the accumulated thickness of oil "layer" on the membrane surface could be estimated as about 1.4 mm. This implies that, during the first 5 seconds, the first layer of oil droplets has not yet formed. Water can easily flow around the oil droplets and go through the membrane, and does not need to penetrate through an oil "layer" on the membrane surface. Back-flushing the membrane once every 5 or 10 seconds may be too frequent to optimise the net flux. According to Srijaroonat et al [30] the optimum filtration and backflush time were 1 minute and 0.7 seconds when using a ceramic membrane for an o/w emulsion filtration.
In the EMBR process, any diethyl sebacate which passes through the membrane and enters the bioreactor will be biodegraded. For further solvent loss reduction, studies using UF membrane for o/w emulsion separation are needed.
4.4 2,4-DCP biodegradation in the MBR
Many bacteria, such as Pseudomonas [32-34], Arthrobacter [35, 36], Rhodococcus, Flavobacterium [37] and Azotobacter [38], etc [39, 40], can utilise CPs as their sole carbon and energy sources. The CPs can be biodegraded via dechlorination of the intermediate after oxidative ring cleavage, dechlorination through hydroxylation or reductive dechlorination [41]. Most engineers prefer mixed cultures, because they are much easier to handle in engineering systems and their synergistic actions help the biodegradation of a mixture of toxic and other compounds [42-45]. In this research, the MBR was inoculated with pond water.
Various aerobic and anaerobic [46, 47] engineering systems have been tested to biodegrade CPs [48]. Some high biodegradation loading rates were reported using fluidised bioreactors (FBR), with a high biomass concentration and good mass transfer [39, 42, 44, 49-51]. When CPs concentration in the FBR was less than 0.1 mg.l-1, a CPs loading rate of 240 g.m-3.day-1 was achieved, and when the CPs concentration in the FBR increased to 1 mg.l-1, the load rate increased to 1,500 g.m-3.day-1 of CPs [51]. Unfortunately, the outlet stream from the FBR was 1 mg.l-1, did not meet the discharge consent. For the EMBR process, the treated water can reach the discharge standard and at the same time, the CPs are biodegraded at a higher concentration in the bioreactor, and so should achieve a higher load rate. The membrane retention of the biomass allows a similar high biomass concentration as in the FBR.
Using a similar flowsheet to that in Figure 4 the following experiment was carried out. Mixed liquor was stored in a seven-litre jacket stainless steel bioreactor with temperature control (Chemap-Fermenter Ltd.). The liquor volume, pH, dissolved oxygen (DO) and temperature of the mixed liquor were closely monitored by electric balance, pH, DO and temperature probes (Mettler Toldedo Ltd. England). A variable speed mixer and the baffle plates inside the bioreactor ensured the liquor was well mixed. Compressed air was filtered through an encapsulated glass micro-fibre filter (Whatman Ltd.) and diffused into the bioreactor through a stainless steel sparger. A condenser was located in the off-gas line to reflux back carried over volatiles. The mixed liquor was circulated by a centrifugal pump (Lowara Ltd. UK.) driven by a frequency converter (Transtronic Ltd. Singapore) and flowed across the two ceramic MF membrane modules (A and B) connected in parallel. The cross flow rates were measured using two turbine flow meters, and the inlet and outlet pressures of the membrane modules and the pressures at the permeate side of the membrane were measured using pressure transducers. A peristaltic pump (Waston-Marlow UK LTD.) pumped the inlet stream into the bioreactor. The constant membrane filtration flux was monitored by a turbine flow meter and regulated by an electric needle valve. A computer controlled the whole system.
The synthetic wastewater with 2,4-DCP of 4,000 mg.l-1 was prepared by adding the following mineral salts to tap water: (NH4)2SO4 (1,200 mg.l-1), MgSO4.7H2O (400 mg.l-1), NaNO3 (100 mg.l-1), Ca(NO3)2.4H2O (200 mg.l-1), FeSO4.7H2O (20 mg.l-1) and yeast extract (1 mg.l-1).
To accelerate the dissolution of 2,4-DCP and to reach a high concentration, 1,000 mg.l-1 potassium hydroxide was added. After the 2,4-DCP was completely dissolved, phosphoric acid was added to neutralise the excessive potassium hydroxide. Potassium di-hydrogen phosphate and di-potassium hydrogen phosphate produced doubled as phosphorous source for bacteria growth and pH buffer. It has been pointed out to the authors subsequently that KOH, whilst providing necessary potassium, might have formed an excess of potassium chloride in the broth with some consequent inhibitory effect. It might be more appropriate to use a mixture of KOH and NaOH in future.
During the initial start-up period, the MBR was run in a semi-batch mode. The high concentration synthetic wastewater was diluted to 30~200 mg.l-1 of 2,4-DCP and each day 3.5 litres of wastewater was fed into the seven litre bioreactor after the same volume of permeate had been drawn out. 1 to 2 hours after starting the feed, the mixed liquor turned to a yellow colour, which faded after 12 to 18 hours. The appearance of this distinct yellow colour has been reported to indicate to the presence of 2-hydroxymuconic semi-aldehyde, one of the intermediates in the metabolic pathway of phenol degradation [52]. Figure 4 gives the total viable bacteria counts in the mixed liquor and the TOC concentrations in the inlet water and permeate from the MBR during this start up period.
The total viable bacteria number in the mixed liquor was quantified by the normal plate count method using nutrient agar CM3 as the culture medium and was incubated at 25 ° C for 36 hours. Direct observation suggested Pseudomonas, rather than Flavobacterium and Rhodococcus, are the dominant bacteria in the MBR. During this experiment, most of the bacteria were growing on the wall of the bioreactor as biofilm, so that the viable bacterial numbers in the mixed liquor in this experiment were much lower than that when a similar MBR system was used for treating ice-cream wastewater [53]. Wall growth phenomena has been widely documented [52, 54, 55] during biodegradation of inhibitory compounds as it gives a region deep within the films where concentrations can be maintained at a low non-inhibitory level.
The differences of TOC concentrations between the inlet water and permeate showed slow adaptation of the culture’s ability to degrade 2,4-DCP. The toxicity of 2,4-DCP to bacteria is obvious when its concentration is high. On day 13 and 14, the viable bacteria numbers in the mixed liquor decreased dramatically, which showed an obvious inhibitory effect of 2,4-DCP but the TOC concentrations in the permeate did not increase accordingly. This confirms the stability of a biofilm to fluctuating 2,4-DCP concentrations. It is worth noting that the baffle plates, tubes and membranes in the MBR offered a quite big surface area for the biofilm. As a biofilm tends to foul the membrane reducing potential fluxes it was desirable to develop an effective suspended culture. Prior work by the authors has shown that suspended bacteria in an MBR was very efficient in treating ice-cream wastewater [53, 56-58].
A new start-up procedure was developed in which the initial carbon source was changed from 2,4-DCP to 2,4-dichlorophenoxyacetic acid (2,4-D), which has a very similar biodegradation pathway to that of 2,4-DCP [59] but which is much less toxic and which has a higher biomass yield. The optical density of the mixed liquor increased quickly with a synthetic wastewater containing 2,000 mg.l-1 of 2,4-D, indicating a rapid growth of the micro-organisms.
After start-up, the MBR was operated in continuous mode. Initially the 2,4-DCP concentration in the inlet water was kept down to 290 mg.l-1 and then slowly increased to 4,000 mg.l-1. The pH and temperature in the bioreactor were kept at 7 ~ 7.4 and 24 ° C ~ 25 ° C respectively.
Although the bioreactor operated with mostly suspended growth during the start-up on 2,4-D once the feed was changed back from 2,4-D to 2,4-DCP, most biomass again became attached to the bioreactor surfaces. Many studies on the biodegradation of toxic compounds have shown that biofilm systems perform better than suspended growth systems [42, 45, 52, 60].
During the experiment, the cross flow speed of the biomass separation was 1.9 m.s-1 and the flux was 4.5 l.m-2.hr-1. Under this condition, the membrane fouling was very slow. After two weeks, the TMP was still too low to be measured, and the pressure drop cross the membrane module was 0.05 bar. It is worth emphasising that in the EMBR process this membrane fouling may be effectively avoided, because the membrane is frequently backflushed by the o/w emulsion filtration. During this period when the membrane is used for receiving the feed from the stripping unit the pH 12 stream is likely to be a very effective cleaner of the membrane surface. On the other hand it may be worth allowing the bioreactor itself to contain extra surface for microbial growth.
The hydraulic retention time in this experiment was 13 hours. Figure 5 shows the 2,4-DCP and TOC concentrations in the inlet water and the permeate from the MBR. 2,4-DCP in the synthetic wastewater was effectively biodegraded. Both 2,4-DCP and TOC concentrations in the permeate were stable, showing that the MBR reached a pseudo-steady state. The average 2,4-DCP concentration in the permeate was 49.9 mg.l-1 and 98.7% of the 2,4-DCP was biodegraded or converted into biodegradable intermediates. The average TOC concentration in the permeate was 237 mg.l-1 and 86.5% of the TOC was biodegraded. The difference between the 2,4-DCP and TOC removal percentage was attributed to presence of intermediate compounds in the permeate. In the EMBR process, these intermediates will enter the striping unit, be extracted by the diethyl sebacate and stripped into the treated water. It has been shown 2,4-DCP is completely biodegradable and the intermediates are less toxic. The chemical oxygen demand (COD) in the treated water from the EMBR process is in a similar range to the discharge standard required. Observation showed that the biomass yield ratio of 2,4-DCP was very low ranging from 0.085 to 0.053 [42].
This experiment showed that when the 2,4-DCP concentration in the bioreactor was about 50 mg.l-1, the MBR operated at a very high 2,4-DCP biodegradation load of 7,300 g.m-3.day-1. This exceeds by 30 times the maximum load reported with an FBR of 240 g.m-3.day-1 [51], when the effluent from the FBR had to be directly discharged to the environment.
Whilst the development of a culture system using halophilic organisms is a possibility one approach to the biodegradation of organic pollutants in the presence of high concentrations of inorganics requires first separating the organic and inorganic components. In some cases silica membranes [62-65], liquid membranes [66, 67] and nano-filtration membranes [68] might be appropriate. Traditional liquid-liquid extraction was suggested for the EMBR process, because the mixer/settler can offer very high stage efficiency, which is crucial to decrease the 2,4-DCP to a very low concentration. Further investigation with a membrane extractor might offer other benefits:
The universal limitation of conventional wastewater treatment is that the discharge consent standards limit the concentrations possible in the bioreactors. These limiting concentrations result in very low biodegradation rates and high reactor volumes. The problem is especially serious where the discharge standards are very low. Many toxic compounds are organic acids or bases, and can dissociate into hydrophilic ions in water, when pH is higher than pKa or lower than pKb. Some of these are shown in Table 1. Among the 129 priority pollutants, phenolic and phthalic acids are acids and amines bases. Using the generic EMBR process, pollutant concentrations in the bioreactor can be much higher than the discharge standard. This increases uptake and reduces reactor volume and cost many fold.
6. Cost Estimates
An estimate has been made of the costs to treat effluent using the above process. As the process was developed with ceramic membranes, and also assuming a less expensive solvent resistant polymer membrane could be used.
Cost basis:
Effluent flow 250 m3d-1 with a CP concentration of 1 kg.m-3.
Acid partition coefficient 1,180, Alkali partition coefficient 0.098.
Ceramic Membranes: $1,800 m-2 installed cost, 20 year lifetime and 60 l.m-2.h-1 average flux
Polymer membranes: $300 m-2 installed cost, $75 m-2 replacement cost, 3 year lifetime and 60 l.m-2.h-1 average flux
Power at 2 kwh.m-2, $ 0.1 kwh-1
NaOH $265 tonne-1
Capital costs for the ceramic process are: Bioreactor $298k; Oil/Water Membrane separation $672k; Waste biomass separation $457k; Extraction units (Total of 6 theoretical stages for the two units) $125k.
A plant life of 20 years and a discount rate of 10 % were assumed. Losses of diethyl sebacate were taken into account. It was assumed that the extracted effluent water did not require neutralisation so that the only caustic used would be within the plant. With these assumptions the total cost of E-MBR treating with the ceramic membrane was $2.81 m-3 effluent, and for the polymeric membrane (assuming a solvent resistant one could be developed) $2.34 m-3 effluent. These costs fall close to $2 m-3, which is a charge typically levied by water utility companies for treating less recalcitrant industrial effluents. It is concluded that the process has a potential in this rather difficult niche market which is being squeezed by advancing regulation.
7. Conclusions
The conceptual EMBR process is a hybrid process combining liquid-liquid extraction, cross-flow microfiltration and a side-loop MBR. It has been shown that for the individual elements of the process:
Liquid-liquid extraction can selectively transfer organic pollutants from a highly salted waste stream into another aqueous stream free of salts. The solvent loss would be very low with o/w emulsion membrane filtration. The extracted organics could then be easily biodegraded.
A side-loop MBR can retain the waste specific biomass in the bioreactor, offering high biodegradation efficiency. The same membrane could be effectively used for both biomass and o/w emulsion filtration alternately from opposite directions. One filtration serves as the backflush of the other, so that membrane fouling is avoided.
Many priority pollutants are organic acids or bases. Their concentrations in the stripping aqueous stream can be increased by pH adjustment. The EMBR process has a major advantage that the pollutant concentrations in the bioreactor are independent of their concentrations in the treated water, thus the biodegradation can be optimised.
The cost of this conceptual process at less than $2.5 m-3 of effluent could be attractive to manufacturers with these specific effluent problems, particularly in comparison with incineration.
Acknowledgement
Wenjun Liu was sponsored by the University of Bath on a Postgraduate Studentship.
References
[1] National Resources Defence Council, Inc. v. Train, 8 ERC 2120, 2122-29 (D.D.C. 1976)
[2] Communication from the commission to the council on dangerous substances which might be included in list I of council directive 76/464/EEC, European Economic Community, 1982
[3] D. T. Gibson, Microbial degradation of organic compounds, Marcel Dekker, New York, (1984)
[4] M. R. Smith, The biodegradation of aromatic hydrocarbons by bacteria, Biodegradation, 1 (1990) 191-206
[5] L. C. M. Commandeur and J. R. Parsons, Degradation of halogenated aromatic compounds, Biodegradation, 1 (1990) 207-220
[6] E. Polnish, H. Kneifel, H. Franzke and K. Hofmann, Degradation and dehalogenation of monochlorophenols by the phenol-assimilating yeast Candida maltosa, Biodegradation, 2 (1992) 193-199
[7] L. A. Golovleva, O. Zaborina, R. Pertsova, B. Baskunov, Y. Schurukhin and S. Kuzmin, Degradation of polychlorinated phenols by Streptomyces rochei 303 Biodegradation, 2 (1992) 201-208
[8] H. H. Tabak, S. A. Quave, C. I. Mashni and E. F. Barth, Biodegradability studies with organic priority compounds, J. Water Pollution Control Federation, 53 (1981) 1503-1518
[9] A. C. Petrasek, I. J. Kugelamn, B. M. Austern, T. A. Pressley, L. A. Winslow and R. H. Wise, Fate of toxic organic compounds in wastewater treatment plants, J. Water. Poll. Cont. Fed., 55 (1983) 1286-1295
[10] W. Gerhartz, Ullmann’s Vol A7 in Encyclopaedia of Industrial Chemistry, VCH publishers, New York, 1986, pp. 3
[11] R. A. Reich and H. J. Cambell, Organics, plastics and synthetics industry discharge: performance and regulations Chemical Engineering Progress, March (1985) 16-21
[12] K. Peys, L. Diels, R. Leysen and C. Vandecasteele, Development of a memberane biofilm reactor for the degradation of chlorinated aromatics, Wat. Sci. Tech., 36 (1997) 205-214
[13] USEPA Method 604, 1982. Research and Development Report 600/4-82-057 Method 8040 1987
[14] W. Liu, Membrane bioreactor for high strength industrial wastewater treatment, Ph.D. thesis, University of Bath, (2000)
[15] D. Mackay and M. Medir, The Application of solvent extraction to waste water treatment, ISEC 77 Proceedings of the International Solvent Extraction Conference, Toronto, September 9th-16th, (1977) 791-797
[16] P. R. Kiezyk and D. Mackay, Waste water treatment by solvent extraction, The Canadian J, of Chemical Engineering, 49 (1971) 747-751
[17] D. S. Abrams and J. M. Prausnitz, Distribution of phenolic solutes between water and non-polar organic solvents, J. Chem. Thermodynamics, 7 (1975) 61-72
[18] K. W. Won and J. M. Prausnitz, Distribution of phenolic solutes between water and polar organic solvents, J. Chem. Thermodynamics, 7 (1975) 661-670
[19] J. P. Earhart, K. W. Won, H. Y. Yong, J. M. Prausnitz and C. J. King, Recovery of organic pollutants from industrial wastewater by solvent extraction, Paper presented at ACS Meeting, San Francisco, August 1976
[20] R. C. Reid, J. M. Prausnitz and B. E. Poling, 1987, Properties of Gases and Liquids McGraw-Hill Book Company, Inc., New York, 1987
[21] J. J. Malinowski and A. J. Daugulis, Salt effects in extraction of ethanol, 1-butanol and acetone from aqueous solutions AIChE J., 40 (1994) 1459-1465
[22] F. A. Long and W.F. McDevit, Activity coefficients of non-electrolytes solutes in aqueous salts solution, Chem. Rev., 51, 119 (1952)
[23] M. Vermue, J. Sikkema, A. Verheul, R. Bakker and J. Tramper, Toxicity of homologous series of organic solvents for the Gram-positive bacteria Arthrobacter and Nocardia Sp. and Gram-negative bacteria Acinetobacter and Pseudomonas Sp., Biotechnology and Bioengineering, 42 (1993) 747-758
[24] A. Inoue and K. Horikoshi, Estimation of solvent-tolerance of bacteria by the solvent parameter Log P. J. Fermentation and Bioengineering 71 (1991) 194-196
[25] D. R. Munro and A. J. Daugulis, The use of an organic solvent and integrated fermentation for improved xenobiotic degradation, Resource and Environmental Biotechnology, 1 (1996) 207-225
[26] R. E. Treybal, Liquid Extraction McGraw-Hill Book Company, Inc., New York, 1963
[27] P. Lipp, C. H. Lee, A. G. Fane and C. J. D. Fell, A fundamental study of the ultrafiltration of oil-water emulsions, J. Membrane Sci., 36 (1988) 161-177
[28] A. B. Koltuniewicz, R. W. Field and T. C. Arnot, Cross-flow and dead-end microfiltration of oily-water emulsion. Part I: Experiment study and analysis of flux decline, J. Membrane Sci. 102 (1995) 193-207
[29] A. B. Koltuniewicz and R. W. Field, Process factors during removal of oil-in water emulsions with cross-flow microfiltration, Desalination 105 (1996) 79-89
[30] P. Srijaroonrat, E. Julien and Y. Aurelle, Unstable secondary oil/water emulsion treatment using ultrafiltration: fouling control by backflushing, J. Membrane Sci., 159 (1999) 11-20
[31] S. Lee, Y. Aurelle and H. Roques, Concentration polarisation, membrane fouling and cleaning in ultrafiltration of soluble oil, J. Membrane Sci. 19 (1984) 23-38
[32] P. M. Radehaus and S. K. Schmid, Characterisation of a novel Pseudomonas sp. that mineralises high concentrations of pentachlorophenol, Appl. & Env. Microbiology, 58 (1992) 2879-2885
[33] H. J. Knackmuss and M. Hellwig, Utilisation and cooxidation of chlorinated phenols by Pseudomonas sp. B 13, Arch. Microbio., 117 (1978) 1-7
[34] C. J. Lu, C. M. Lee and C. H. Huang, Biodegradation of chlorophenols by immobilised pure-culture microorganisms, Wat. Sci. Tech. 34 (1996) 67-72
[35] G. J. Stanlake and R. K. Finn, Isolation and characterisation of pentachlorophenol-degrading bacterium, Appl. & Env. Microbiology, 44 (1982) 1421-1427
[36] C. Hagedorn and J. G. Holt, A nutritional and taxonomic survey of Arthrobacter soil isolates. Can. J. Microbiol., 21 (1975) 353-361
[37] D. L. Saber and R. L. Crawford, Isolation and characterisation of Flavobacterium strains that degrade pentachlorophenol, Appl. & Env. Microbiology, 50 (1985) 1512-1518
[38] D. Y. Li, J. Eberspächer, B. Wagner, J. Kuntzer and F. Lingens, Degradation of 2,4,6-trichlorophenol by Azotobacter strain GP1. Appl. & Env. Microbiology, 57 (1991) 1920-1928
[39] J. A. Puhakka, R. P. Herwig, P. M. Koro, G. V. Wolfe and J. F. Ferguson, Biodegradation of chlorophenols by mixed and pure cultures from a fluidised-bed Reactor, Appl. Microbiol Biotechnol., 42 (1995) 951-957
[40] J. G. Steiery, W. J. Thoma, K. Ugurbil and R. L. Crawford, 31P Nuclear magnetic resonance studies of effects of some chlorophenols on Escherichia coli and a pentachlorophenol degrading bacterium, J. Bacteriology, 170 (1988) 4954-4957
[41] M. Haggblom, Mechanisms of bacterial degradation and transformation of chlorinated monoaromatic compounds, J. Basic Microbiol., 30 (1990) 115-141
[42] J. A. Puhakka and K. Järvinen, Aerobic fluidised-bed treatment of polychlorinated phenolic wood preservative constituents, Wat. Res., 26 (1992) 765-770
[43] H. S. Bae, J. M. Lee and S. T. Lee, Biodegradation of the mixture of 2,4,6-trichlorophenol, 4-chlorophenol, and phenol by a defined mixed culture J. Gen. Appl. Microbiol., 43 (1997) 97-103
[44] J. A. Puhakka, E. Melin, K. Järvinen, T. Tuhkanen and W. K. Shieh, Oxic fluidised-bed treatment of dichlorophenols, Wat. Sci. Technol., 24 (1991) 171-177
[45] E. S. Melin, J. A. Puhakka and J. F. Ferguson, Enrichment and operation strategies for polychlorophenol degrading microbial cultures in an aerobic fluidised-bed reactor, Water Environment Research, 70 (1998) 171-180
[46] S. A. Body and D. R. Shelton, Anaerobic biodegradation of chlorophenols in fresh and acclimated sludge, Applied and Environmental Microbiology, 47 (1984) 272-277
[47] P. Jin, S. K. Bhattacharya, Toxicity and biodegradation of chlorophenols in anaerobic propionate enrichment culture, Water Environment Research, 69 (1997) 938-947
[48] A. P. Annachhatre and S. H. Gheewala, Biodegradation of chlorinated phenolic compounds, Biotechnology Advances, 14 (1996) 35-56
[49] P. M. Makinen, T. J. Theno, J. F. Ferguson, J. E. Obgerth and J. A. Puhakka, Chlorophenol toxicity removal and monitoring in aerobic treatment: recovery from process upsets, Environ. Sci. Technol. 27 (1993) 1434-1439
[50] E. S. Melin, K. T. Järvinen and J. A. Puhakka, Effects of temperature on chlorophenol biodegradation kinetics in fluidised-bed reactors with different biomass carriers, Wat. Res., 32 (1998) 81-90
[51] K. Järvinen and J. A. Puhakka, Bioremediation of chlorophenol contaminated ground water, Environmental Technology, 15 (1994) 823-832
[52] G. Molin and I. Nilsson, Degradation of phenol by Pseudomonas putida ATCC 11172 in continuous culture at different ratios of biofilm surface to culture volume, Appl. Environ. Microbiol. 50 (1985) 946-950.
[53] J. A. Scott and K. L. Smith, A bioreactor coupled to a membrane to provide aeration and filtration in ice-cream factory wastewater remediation, Wat. Res., 31 (1997) 69-74
[54] L. D. Collins and A. J. Daugulis, Biodegradation of phenol at high initial concentrations in two-phase partitioning batch and fed-batch bioreactors, Biotechnology and Bioengineering, 55 (1997) 155-162
[55] L. D. Collins and A. J. Daugulis, Use of two phase partitioning bioreactor for the biodegradation of phenol, Biotechnology Techniques, 10 (1996) 643-648
[56] J. A. Scott, D. J. Neilson, W. Liu and P. N. Boon, A dual function membrane bioreactor system for enhanced aerobic remediation of high strength industrial waste, Water Science and Technology, 38 (1998) 413-420
[57] J. A. Scott, J. A. Howell, T. C. Arnot, K. L. Smith and M. Bruska, Enhanced system kLa and permeate flux with a ceramic membrane bioreactor, Biotechnology Techniques 10 (1996) 287-290
[58] J. A. Scott and W. Liu, A dual-function twin-membrane bioreactor for high-rate aerobic remediation of organic waste streams. Proc. Env. Strategies for the 21st Century, Univ. Singapore Press, 1998, pp. 421-426
[59] W. C. Evans, B. S. Smith, H. N. Fernley and J. I. Davies, Bacterial metabolism of 2,4-Dichloropheoxyacetate, Biochem. J., 122 (1971) 543-511
[60] H. J. Heipieper, H. Keweloh and H. J. Rehm, Influence of phenols on growth and membrane permeability of free and immobilised Escherichia coli., Applied and Environmental Microbiology, Apr. (1991) 1213-1217
[61] D. D. Beer and P. Stoodley, Relation between the structure of aerobic biofilm and transport phenomena, Wat. Sci. Tech. 32 (1995) 11-18
[62] A. J. Livingston, A novel membrane bioreactor for detoxifying industrial wastewater: I. Biodegradation of phenol in a synthetically concocted wastewater, Biotechnology and Bioengineering, 41 (1993) 915-926
[63] A. J. Livingston, A novel membrane bioreactor for detoxifying industrial wastewater: II. Biodegradation of 3-chloronitrobenzene in an industrially produced wastewater, Biotechnology and Bioengineering, 41 (1993) 927-936
[64] P. Pavasant, L. M. F. dos Santos, E. N. Pistikopoulos and A. G. Livingston, Prediction of optimal biofilm Thickness for membrane-attached biofilms growing in an extractive membrane bioreactor, Biotechnology and Bioengineering, 52 (1996) 373-386
[65] L. F. Strachan and A. G. Livingston, The effect of membrane module configuration on extraction efficiency in an extractive membrane bioreactor, J. of Membrane Sci., 128 (1997) 231-242
[66] T. Kataoka, T. Nishiki, K. Osaki and A. Muto, A practicable process for phenol removal with liquid surfactant membrane permeation column, Separation Science and Technology, 32 (1997) 1447-1462
[67] T. Kakoi, M. Goto, S. Natsukawa, K. Ikemizu and F. Nakashio, Recovery of phenols using liquid surfactant membrane prepared with newly synthesised surfactants, Separation Science and Technology, 31 (1996) 107-124
[68] M. Nyström, L. Kaipia and S. Luque, Fouling and retention of nanofiltration membranes, J. Membrane Sci., 98 (1995) 249-262
Figure titles:
Figure 1: Schematic Diagram of the EMBR Process.
Figure 2: 2,4-DCP’s distribution between diethyl sebacate and water at different pH.
The points are experimental data, the line corresponds to Equation 5.
Figure 3: Schematic of Dual-membrane Bioreactor.
Figure 4: Feed and permeate TOC levels, and bacterial counts in the batch operation of MBR.
Figure 5: 2,4-DCP Biodegradation in Continuous MBR Operation.
Table 1: The pKa of some selected pollutants* which would be amenable to treatment
using this process.
Compound pKa [29]
phenol 9.89
2-chlorophenol 8.52
2,4-dichlorophenol 7.90
2,4,6-trichlorophenol 5.99
pentachlorophenol 4.74
2-nitro phenol 7.17
2,4-dinitro phenol 2.15
aniline 4.63
2,4-dichloroaniline 2.05
o-phthalic acid 2.89
m-phthalic acid 3.54
p-phthalic acid 3.51
* The latter 3 are not on the red list, but some of their breakdown products, such as dimethyl phthalate and diethyl phthalate, are.
Table 2: Diethyl sebacate (DS) concentrations in the permeate stream for different
filtration conditions.
|
TMP of filtration (bar) |
TMP of backflush (bar) |
Backflush frequency (s-1) |
DS in permeate (mg.l-1) |
|
0.1 |
0.1 |
0.2 |
< 0.1 |
|
0.2 |
0.2 |
0.2 |
0.8 |
|
0.3 |
0.3 |
0.2 |
1.7 |
|
0.1 |
0.1 |
0.1 |
0.1 |
|
0.1 |
0.1 |
0.067 |
1.0 |
|
0.1 |
0.1 |
0.05 |
3.0 |