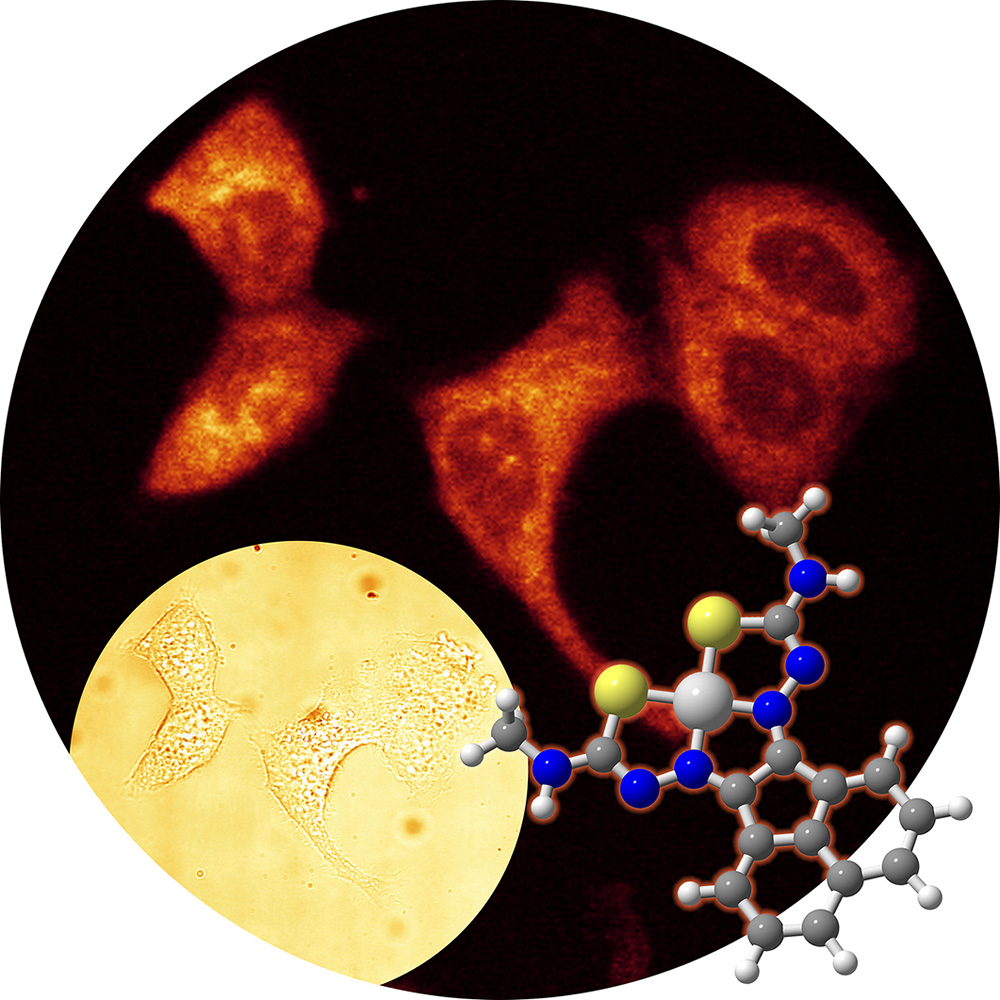
 Professor Sofia I.
Pascu FRSC FHEA Professor Sofia I.
Pascu FRSC FHEA
Professor in Bioinorganic and Materials Chemistry, University of Bath
Academic Visitor, Oxford Siemens Molecular Imaging Laboratory, Inorganic Chemistry Laboratory, University of Oxford
Main research interests: Metals in medicine, homegeneous- and nano-catalysis and synthetic functional materials
- Multimodal PET/optical molecular imaging techniques: Targeted delivery of metallodrugs, encapsulated within Single
Wall Carbon Nanotubes, Dynamic Nanohybrids and Nanocapsules
- Designing new radiopharmaceuticals for PET or SPECT imaging and/or therapy; radiolabelling methods with Cu-64, Ga-68 and Zr-89
- Multiphoton fluorescence imaging techniques: confocal fluorescence and lifetime fluorescence imaging (FLIM) of metallic species in cancer cells
- Pseudorotaxane-type structures for molecular switches
- Synthetic coordination and organometallic chemistry with sustainable chemistry applications
- Homogeneous catalysis using tripodal N-heterocyclic carbenes, i.e. Pd(II) and Cu(I), for C-C coupling reactions such as Sonogashira, Suzuki and Heck reactions
- Functional materials for Fischer Tropsch chemistry and Sustainable Chemistry Applications
- Single crystal X-ray diffraction using synchrotron radiation
- Designing light emitting hybrid nanomaterials and donor-acceptor supramolecular complexes for photovoltaic applications
- Covalent and non-covalent functionalisation of graphene, graphene oxide, carbon nanoparticles and single walled carbon nanotubes
Targeted delivery of metal ions encapsulated within single
wall carbon nanotubes and dynamic nanocapsules
Several drug delivery and targeting strategies have been developed
that are based on coupling drugs to receptor-specific ligands
and/or protection of the drug by wrapping it in a polymer or
lipid coat. We insert metallic ions such as Cu radioisotopes
into more kinetically stable transport systems and then guide these to the target
by conjugation to an appropriate targeting biomolecule.
‘Hot’’ complexes or ionic materials will be
encapsulated inside carriers, i.e. single wall carbon nanotubes SWNTs
(a), or nanocapsules (b). These assemblies
are derivatised with groups able to modulate the lipophilicity
(e.g. carboxylate units, also suitable for further synthetic
modifications), to provide biological targeting (i.e. an address,
which is an antibody or related biological molecule incorporating
recognition motifs, linked covalently or non-covalently to the
main ‘carrier’) or to provide a fluorescent tag (e.g.
dansyl chloride, nitrobenzaoxadiazoles for monitoring the delivery
process and cell uptake studies).
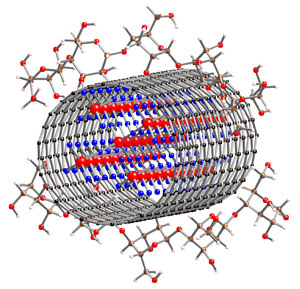
a) SWNTS as carriers for ‘hot’ metal ions – collaboration
with Oxford Nanotube Group and Oxford Siemens Molecular Imaging Laboratory
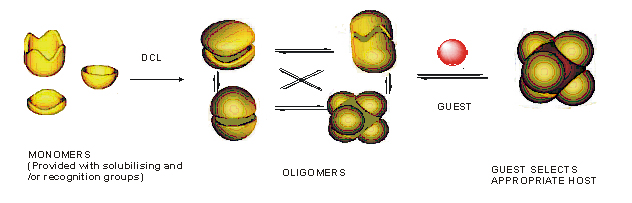
b) Formation of nanocapsules as hosts for ‘hot’ metal
ions – collaboration with Dr Sijbren Otto (Cambridge)
Designing new Cu-64 radiopharmaceuticals for PET imaging and
therapy
Positron emission tomography
(PET) will be one of the primary tools for the diagnosis of cancer
and for the monitoring of the effects of therapy. Therefore,
there is a great interest in developing new target-specific radiopharmaceuticals.
This synthetic programme identifies target complexes to be synthesised,
cell delivery and targeting strategies, and methods for testing
these in biological systems. Copper-64 is both a beta and positron
emitter, offering the possibility of simultaneous imaging and
therapy. The relatively long half-life of 64Cu (12.7
hours) makes it an attractive isotope for PET imaging since it
can carried out on a site remote from the cyclotron used to generate
the radionuclide. Despite this there are very few
available bifunctional copper chelators (based on nitrogen, phosphorus
and oxygen donors) with optimal physical characteristics in terms
of neutral charge, redox and kinetic stability. While based on
known coordination chemistry principles, the successful synthesis
of ‘hot’ complexes will represent a significant step
forward in the use of Cu-based imaging agents. This work is currently
carried out in collaboration with Professor Jon Dilworth and
his research group within CRL. Radiolabelling studies
are carried out in collaboration with the Centre for Molecular
Imaging of Functional Biological Systems, Oxford as well as Addenbrooke’s
Hopsital (Wolfson Brain Imaging Centre, led by Dr Frank Aigbirhio). Feedback from the testing is
incorporated into optimising the complex design.
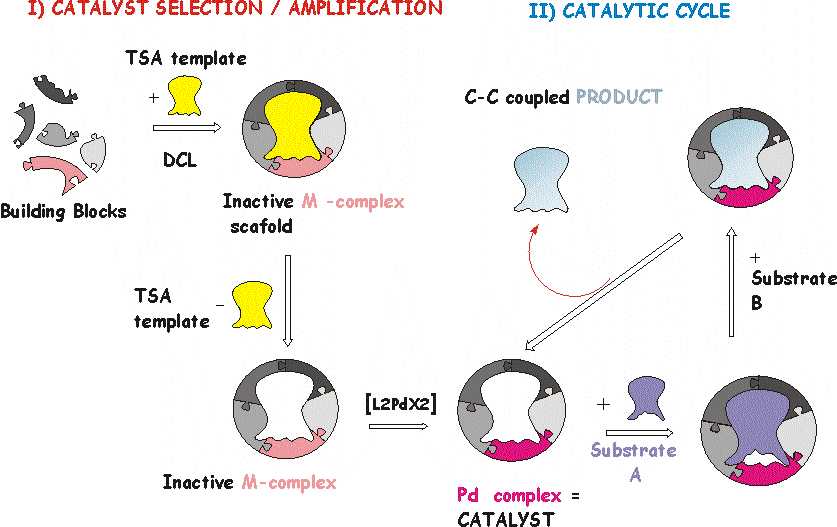
Supramolecular homogeneous catalysis
The development of homogeneous
catalysts that rival the efficiency and selectivity of enzymes
is one of the holy grails in chemistry. My objective is to use
dynamic combinatorial chemistry (DCC) to discover highly selective
homogeneous metallic catalysts. This overcomes problems of prior
synthetic approaches, in which the “trial and error” synthesis
of selective catalysts is rarely successful and immensely time-consuming.
The DCC strategy to generate specific receptors has already been
making an impact in academia, but this research has not yet been
extended to homogeneous transition metal catalysis of processes
of industrial relevance. This work is sponsored by a Royal Society Joint Collaboration Grant, with Professor Makoto Fujita
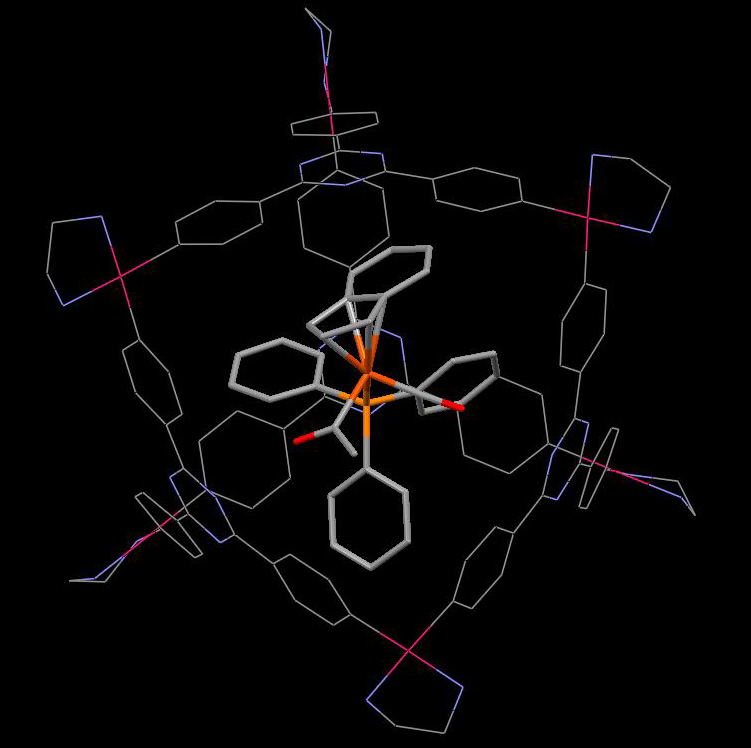
Pseudorotaxane-type structures for molecular switches
One of
the key goals of supramolecular chemistry is to assemble structural
building blocks into arrays with new properties that emerge only
in supramolecular architectures. Rotaxanes and catenanes have
for some time been the subject of intense study both because
of their fascinating architectures and because they may have
useful properties as molecular level switches and sensors. We
are interested in applying this fundamental knowledge to develop
molecular assemblies that can perform tasks analogous to the
machines of everyday life.
My approach
has been to bring together neutral electron-rich components and
electron-poor components to create donor–acceptor systems
that are neutral, chemically robust and capable of post-synthetic
modification. These weak non-covalent interactions can be reinforced
under the effect of alkali salts. This work is in collaboration with Prof Jeremy Sanders (Cambridge).
Synthetic coordination and organometallic chemistry
My general objectives are to understand the intimate mechanisms
of important catalytic reactions and to discover and study new
catalytic processes. My recent work has been concerned with late
TM catalysts for C-C coupling reactions, and with establishing
new Fe(II)-based organometallic systems active in homogeneous
Fischer-Tropsch (FT) catalysis.
Single crystal X-ray diffraction using synchrotron radiation
A vital component of my work is to characterise the complexes
synthesised and their precursors crystallographically. Redox-related
changes in biological properties of copper-bis(thiosemicarbazone)
radiopharmaceuticals such as hypoxia tracer diacteylbis(4-methyl-3-thiosemicarbazonato)copper(II)
Cu(ATSM) are mediated by changes in core structural parameters.
A good understanding of single crystal X-ray structures provides
considerable insight into solid state packing interactions, which
play a significant part in determining fluorescence lifetimes
and efficiencies of the compounds of interest.
Many of the single crystal structures are amenable to solution
using the laboratory-based equipment in Bath, but a large number
of inorganic and organometallic precursors afford only small
weakly diffracting single crystals. Additional X-ray flux available
on Station 9.8 was necessary to provide good quality data.
As a trained SRS user, I carried out the experimental work at Daresbury
and all structural analysis. Collaborative support was provided
by Prof Paul Raithby (Bath) and Dr John Warren (SRS Daresbury/Bath).
Selected Publications
- S. I. Pascu*, N. Kuganathan , L. H. Tong , R. M. J. Jacobs, P. J. Barnard , B. T. Chu , Y. Huh, G. Tobias , C. G. Salzmann , J. K. M. Sanders, M. L.H. Green, J. C. Green,
Interactions between Tripodal Porphyrin Hosts and Single Walled Carbon Nanotubes: an Experimental and Theoretical (DFT) Account
J. Mater. Chem., 2008,18, 2781.
- S. I. Pascu*, P. A. Waghorn, T. D. Conry, B. Lin, H. M. Betts, J. R. Dilworth, R. B. Sim, G. C. Churchill, F. I. Aigbirhio, J. E. Warren ,
Cellular Confocal Fluorescence Studies of the Cytotoxic Activity of New Zn(II) Bisthiosemicarbazonato Complexes
Dalton Trans., 2008, 2107.
- S. I. Pascu*, P. A. Waghorn , T. D. Conry, H. M. Betts, J. R. Dilworth, G. C. Churchill, T. Pokrovska, M. Christlieb ,
F. I. Aigbirhio, J. E. Warren ,
Designing Zn(II) and Cu(II) derivatives as probes for in vitro fluorescence imaging
Dalton Trans., 2007, 4988.
- S. I. Pascu *, C. Naumann , Guido Kaiser, Andrew Bond, J. K. M. Sanders, T. Jarrosson,
Structures and solution dynamics of cation-mediated pseudorotaxanes
Dalton Trans.,2007, 3874.
- Z. Rodriguez-Docampo, S. I. Pascu, S. Kubik, and S.Otto,
Noncovalent interactions within a synthetic receptor can reinforce guest binding
J. Am. Chem. Soc., 2006, 128(34), 11206.
- L. H. Tong, S. I. Pascu, T. Jarrosson, and J. K. M. Sanders,
Large-scale synthesis of alkyne-linked tripodal porphyrins via Palladium-mediated coupling conditions
Chem. Commun., 2006, 1085.
- A. L. Kieran, S.
I. Pascu, T. Jarrosson, M. J. Gunter and
J. K. M. Sanders ,
Dynamic synthesis of a macrocycle containing a porphyrin and an electron donor Chem. Commun., 2005,
1842.
- A. L. Kieran, S. I. Pascu, T. Jarrosson, and J. K. M. Sander Inclusion of C60 into
an adjustable porphyrin dimer generated by dynamic disulfide
chemistry Chem. Commun., 2005, 1276.
- S. I. Pascu, T. Jarrosson, C. Naumann, S. Otto, J. K. M. Sanders Cation-reinforced donor-acceptor
pseudorotaxanes New J. Chem., 2005,
80.
- S. L. J. Conway, M. L. H. Green*, S. I. Pascu* and H. O. Peake Structure-reactivity correlations in new
W(IV) and Nb(IV) silicon-bridged ansa-metallocene
hydrides Polyhedron 2005, 25, 406.
- J. R. Dilworth, C. A. Maresca von Beckh W. and S. I. Pascu Synthesis, Structures and Catalysis of Thioether-Phosphane
Complexes of Pd(II) and Pt(II) Dalton Trans., 2005,
2151.
- S. I. Pascu*, K. S. Coleman,
A. R. Cowley, M. L. H. Green* and N. L. Rees New Cationic Palladium(II)
and Rhodium(I) Complexes of [Ph2PCH2C(Ph)=N(2,6-Me2C6H3)] J.
Organomet. Chem., 2005, 690, 1645.
- G. D. W. Anderson, O. J. Boys, A. R. Cowley, J. C. Green,
M. L. H. Green, S. A. Llewellyn, C. Maresca von Beckh, S. I.
Pascu, I. C. Vei Structural Investigations on New
Iron-acyl Derivatives of B(C6F5)3 J.
Organomet. Chem., 2004, 689, 4407.
- I. C. Vei, S. I. Pascu, M. L. H. Green, J. C. Green, R.
E. Schilling, G. D. W. Anderson, L. H. Rees Synthesis and study
of binuclear compounds containing bridging (m-CN)B(C6F5)3 and
(m-NC)B(C6F5)3 systems Dalton
Trans., 2003, 2550.
|
|