
Atomistic simulation is well suited to the calculation of the structure and relative stability of a whole range of ceramics. However, the high temperatures and pressures found within the Earth make the use of simulation even more appealing because experiments are often difficult and subject to a high degree of error.

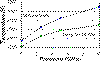
We have simulated, using molecular dynamics, the effect of temperature and
pressure on the fast ionic conductivity of such an isostructural analogue; KCaF3.
The effect of pressure results in a decrease in the
critical temperature for conduction relative to the melting point indicating
that conductivity may be possible deep within the mantle.
MgSiO3 perovskite is thought to be the dominant phase of the Earth's lower
mantle and hence accounts for approximately 40% of the earth by volume.
The conductivity of the material has been investigated by experiment and
simulation although the experiments are subject to large error and competing
experimental groups have not been able to agree on their conclusions. An
alternative is to study analogue fluorides which require much lower temperatures
and lower pressures to produce the same volume contraction and to melt.
 Ionic Conductivity of a Structural Analogue of MgSiO3
Ionic Conductivity of a Structural Analogue of MgSiO3
(3K) |
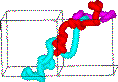
In addition, animation of the trajectories of the atoms allows prediction of the diffusion mechanism. This is shown below to result from a concerted hopping motion for perovskite.
(17K) |
related references:

Simulations using lattice dynamics indicate that the quartz structure
becomes dynamically unstable at 21.5 GPa. Animation (shown by the Java Applet
below) of this mode indicates
that the channels in quartz rotate in cooperation.
Quartz is the stable form of SiO2 up to approximately 3 GPa and is
metastable up to 15-25 GPa. At these higher pressures it becomes amorphous
and the mechansim for which is not well understood.
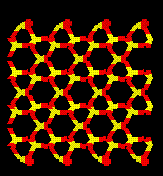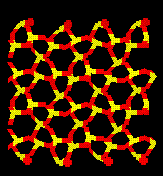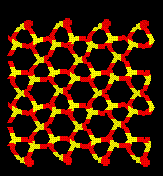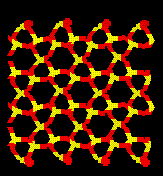
 Pressure Induced Amorphization of Quartz.
Pressure Induced Amorphization of Quartz.
|
Relaxation of cells which have had the ions displaced along the eigenvectors of motion for this dynamically unstable mode result in collapse of the crystal structure. Below is a picture of pure quartz and a typical amorphous cell.
related references:

Real materials are generally polycrystalline and the material
properties can be significantly modified by the presence of grain boundaries.
When considering mantle forming minerals these boundaries become important
since they provide a means for creep and ion migration, likely causes of mantle
rheology.
 Structure and Stability of Grain Boundaries.
Structure and Stability of Grain Boundaries.
One such mineral is Periclase (MgO) which makes up 10% of the lower mantle.
Three tilt grain boundaries have been modelled using lattice dynamics. Pictures
of these are given below.
The formation energies of these boundaries increase with the decrease in the density of cross linking bonds.
Further work in this area will consider the effects of pressure and temperature upon the formation energy in order to simulate realistic mantle conditions and diffusion of ions both along and across the boundary.
related references:

Computational solid state chemistry group home page
page design by Graeme Watson and Pete Oliver.
Send your comments and questions to
s.c.parker@bath.ac.uk.
