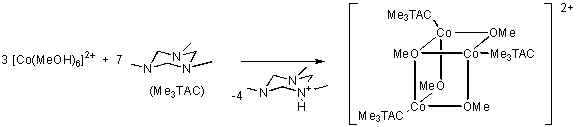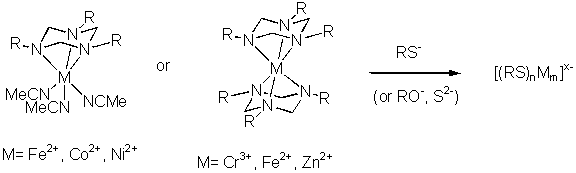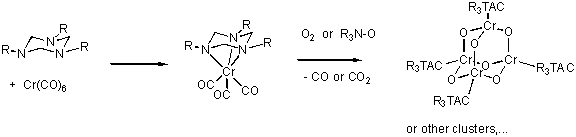Research Opportunities in our group
If you are interested in any of the proposed projects, contact
Dr. Randolf Köhn
for postgraduate studentships
Proposed Research Areas:
- Synthesis of Cr/O-Clusters
- Copper Complexes as Bio-analogous Model Complexes
-
Catalytical Polymerisation and Selective Trimerisation of Olefines with Chromium Complexes
- Triazacyclohexane Complexes of Titanium and Lanthanoids as Lewis Acid Catalysts
Investigations towards the Biological Role of Chromium:
Chromium has been known to be an essential element for over 50 years.
However, only recently the actual biological role of this metal becomes known.
Apparently, Cr/O clusters of up to four chromium atoms can increase the activity of
insulin by binding to a small chromium binding protein. Thus, chromium may be an important
metal in the glucose metabolism. A study suggests that up to 90% of all Americans do not absorb
sufficient amounts of chromium from their diet and this may be related to the development of some
forms of diabetes. It is yet unknown what form of chromium is required as effective yet non-toxic
food supplement. Recent discoveries have shown some potential for simple clusters like
[Cr3O(propionate)3]+.
This project involves the syntheses of new triazacyclohexane capped clusters of chromium and other transition metals.
Simple tests for their activity can be performed in collaboration with Prof. Holman from the Biochemistry Department.
Additional studies will involve their potential use as metal delivering drugs.
The clusters will be characterized by various spectroscopic techniques.
more details...
Investigation of Copper Complexes as New Models for Biological Oxygen Activation or Transport Systems:
The synthesis of new copper complexes and the mechanistic investigation of their reaction remains
an important area of bioinorganic chemistry as they may serve as model systems of dioxygen
binding and activating metalloproteins. Especially the in-depth study of the copper(I) complexes of
larger triazacycloalkanes (ring size 9-12) with various N-substituents has yielded important insights
of the factors influencing the formation of peroxo- or bis(oxo)-bridges and the subsequent oxidation
of C-H-bonds.[1]
Generally, the activation of O2 by the copper(I) complexes via the formation of
bis(oxo)-Cu(III) complexes is increased by increasing exposure of the copper atom
(from the 12-membered ring-ligand to the 9-membered) and the increased tendency to form a square-pyramidal
N3CuO2 (sum of the N-Cu-Cu angles S=270°)
coordination geometry rather than a quasi tetrahedral N3Cu(O2)
geometry (S=330°).
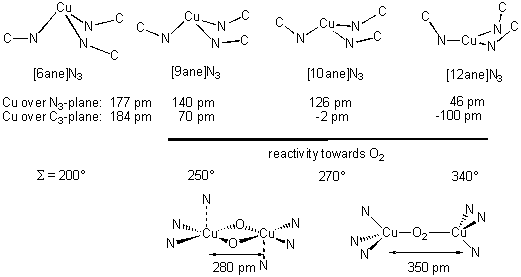
The large angle sum S, deeply "burried" Cu and bulky N-substituents keeping
the two Cu moieties apart favour no or only weak (reversible) coordination of dioxygen while small
S, exposed Cu and low steric bulk favour oxygen activation.
Previous structural studies in our group have shown that analogous copper(I) complexes of
triazacyclohexanes can be prepared and that the copper is much more "exposed" and that S
is much smaller than in the larger triazacycloalkane complexes.[2]
Thus, they should be more reactive towards
oxygen and should easily form bis(oxo) bridged complexes. We propose to study the reaction of triazacyclohexane
copper(I) complexes with dioxygen in comparison to the study of the larger triazacycloalkanes.
It should be possible to identify the initial reaction product by UV/vis and Raman spectroscopy at low temperature.
We have recently obtained fibre optical UV/vis and Raman spectrometers for other projects. These should be ideally
suited to study this reaction. Studies of the site of the C-H activation on the highly reactive triazacyclononane
complexes usually show intra-molecular hydrogen abstraction from the neighbouring N-substituent as the first step.
The triazacyclohexane complexes may offer the additional advantage that the N-substitents are much farther away from
the Cu2O2-core due to the large Cu-N-alkyl angle (see above).
Thus, the initial site of oxidation may become the solvent instead of the ligand and intra-molecular substrate oxidation
may become possible. In this study, we also want to look for oxidation products to support this possibility.
- B. M. T. Lam, J. A. Halfen, V. G. Young, Jr., J. R. Hagadorn, P. L. Holland, A. Lledós, L. Cucurull-Sánchez, J. J. Novoa, S. Alvarez, and W. B. Tolman,
Inorg. Chem. 39 (2000) 4059-4072; and references therein.
- Strained 1,3,5-Triazacyclohexane Complexes of Copper(I) and (II),
R. D. Köhn, G. Seifert, G. Kociok-Köhn,
Chem. Ber., 129 (1996) 1327-1333.
New Chromium Based Catalysts for the Polymerisation and Trimerisation of Ethylene:
We have recently discovered that triazacyclohexane complexes of CrCl3 can be activated with
MAO to give highly active catalysts. A large variety of different triazacyclohexanes can be prepared
from primary amines and formaldehyde. The activity and selectivity for polyethylene or 1-hexene as products
can be strongly influenced by little changes in the amine, especially in the 2-position.
The project will involve the synthesis and characterization of new analogous complexes from other amines
and the testing of their activity towards ethylene. The project will involve simple organic and coordination chemistry
as well as performing ethylene polymerizations under highly inert conditions.
Cp*TiCl3/MAO (methyl aluminoxane) is (so far) the only alternative to chromium based catalysts
in their ability to trimerise olefins selectively. Analogous to the chromium systems,
they react with olefins via Ti(II) complexes and Ti(IV) metallacycles.
In our studies on chromium complexes, we found that replacement of the anionic Cp by neutral triazacyclohexane ligands greatly
improved the selectivity and activity. We have recently synthesized analogous titanium and lanthanoid complexes,
e.g. [(triazacyclohexane)TiCl3]+ or
[(triazacyclohexane)2Pr(OTf)3].
Triazacyclohexanes can easily be prepared from primary amines and formaldehyde.
In this project, new early transition metal complexes will be synthesized and tested for their catalytic activity.
The compounds will be characterised by NMR and X-ray crystallography.
In earlier experiments we have been able to prepare complexes with the open cubane type core
Co3O4 stabilised by triazacyclohexane ligands.[1]
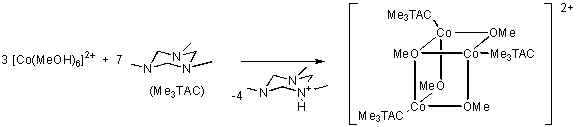
In these complexes the cobalt reaches co-ordination number 6 while most cubane-type clusters
have co-ordination number 4 which makes them more susceptable to ligand exchange reactions.
This indicates that it might be possible to use triazacyclohexanes for the stabilisation of transition
metal clusters with MnXm (X= O, S) cores in general. We also found that the NMR signals for the
triazacyclohexane in these paramagnetic compounds are well resolved and shifted over a large range
and that they are quite sensitive to little changes in the environment such as the solvent or counter anion.
Thus, the rigidly co-ordinated triazacyclohexanes may also be of use as NMR probes in paramagnetic transition
metal clusters. Many (often paramagnetic) transition metal clusters play an important role in biological systems,
e.g. FenSm in redox enzyms, Mn4Om in the photosystem II or more recently
Cr4Om in the LMWCr as the biologically active form of chromium which may play an important role
in the amplification of insuline in the sugar metabolism.[2]
In previous unpublished work we have prepared several triazacyclohexane complexes that should be suitable precursors
for the synthesis of such clusters by substitution of labile ligands (e.g. MeCN or a second triazacyclohexane):
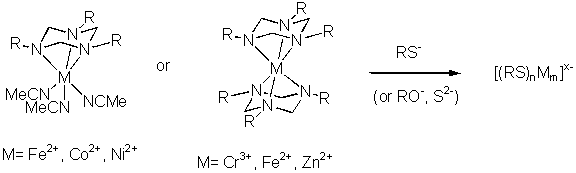
Alternatively, the "X" atoms in the clusters could be introduced by oxidation of low valent triazacyclohexane carbonyl
complexes. The complexes [(triazacyclohexane)Cr(CO)3] are well known [3] and can easily be prepared by heating
Cr(CO)6 with the ligand. Earlier studies have shown that these complexes can be oxidised by chlorine, bromine
(to Cr(III)) or even H2O2 (to Cr(VI)) under conservation of the coordinated triazacyclohexane.
In this study, the synthesis of analogous carbonyl complexes of Mn, Fe or Co and subsequent oxidation with suitable
O- or S-transfer reagents (e.g. R3NO or O2 or S8 ) could lead to
[(triazacyclohexane)nMnX2m] complexes. [(triazacyclohexane)Cr(CO)3]
is known to be oxidised by air to green hydrocarbon soluble products that have not been identified so far.
Controlled oxidation may well give the desired cluster compounds, e.g.:
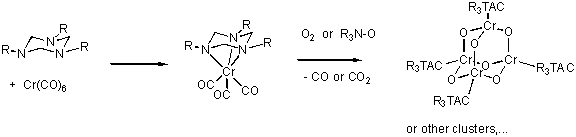
-
Synthesis and Characterization of the Cobalt(II) Methoxide Core {Co3(OMe)4}2+,
R. D. Köhn, M. Haufe, G. Kociok-Köhn, A. C. Filippou,
Inorg. Chem. 36 (1997) 6064-6069.
-
Y.J. Sun, J. Ramirez, S.A. Woski, J.B Vincent, J. Biol. Inorg. Chem. 5 (2000) 129-136;
Y.J. Sun, K. Mallya, J. Ramirez, J.B. Vincent, J. Biol. Inorg. Chem. 4 (1999) 838-845;
J.B. Vincent, Acc. Chem. Res. 33 (2000) 503-510; and references therein.
-
M.V. Baker, D.H. Brown, B.W. Skelton, A.H. White, J. Chem. Soc. Dalton Trans. (2000) 763-768;
M.V. Baker, M.R. North, B.W. Skelton, A.H. White, Inorg. Chem. 38 (1999) 4515-4521; and references therein.