20. Taxane Diterpenes 5: Synthesis of the a- and C-Rings: An Unusual Rearrangement of an N-Hydroxyimino Lactone
Tetrahedron 1999, 55, 6435-6452

Magnus, P.; Westwood, N.; Spyvee, M.; Frost, C.; Linnane, P.; Tavares, F.; Lynch, V.
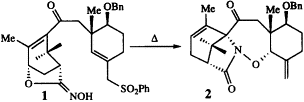
Ring A of taxol was synthesized from the bromodiene 5 and acryloyl chloride to give 6, which was resolved by separation of diastereomers 7. Allylic oxidation of 7 gave 8, which on deprotection, esterification and reduction gave 11. Heating 11 followed by reduction and protection gave 13. The C-ring component was made using asymmetric Birch reduction methodology combined with standard functional group manipulations to give 28. Combining 13 and 28 gave 29, which was converted into 33. Thermolysis of 33 did not generate a nitrile oxide but rather ionized to an oxydienyl cation, eventually leading to 34.

