59. Rhodium-Catalyzed Conjugate Addition-Enantioselective Protonation: The Synthesis of alpha, alpha'-Dibenzyl Esters
Organic Letters 2007, 9(11), 2119-2122

Christopher G. Frost, Stephen D. Penrose, Kim Lambshead, Paul R. Raithby, John E. Warren, and Robert Gleave
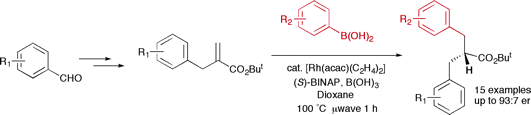
alpha-Benzyl acrylates, which are conveniently prepared from the corresponding aldehydes, can be employed as substrates in a tandem rhodium-catalyzed conjugate addition-enantioselective protonation protocol to afford enantiomerically enriched alpha,alpha'-dibenzyl esters. The synergistic effect of enantiopure ligand and proton source was rapidly optimized with use of a microwave reactor.

